Sandbox Reserved 426
From Proteopedia
| Line 19: | Line 19: | ||
==Binding Interactions== | ==Binding Interactions== | ||
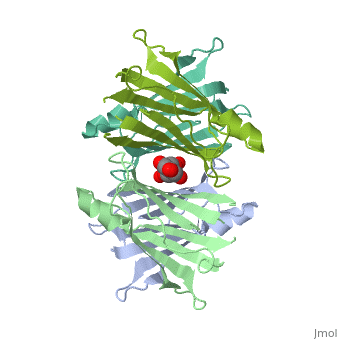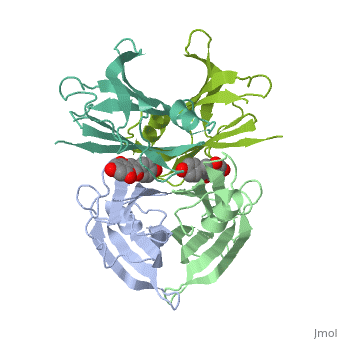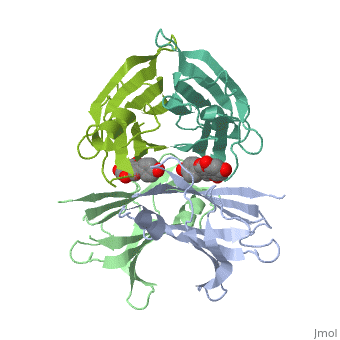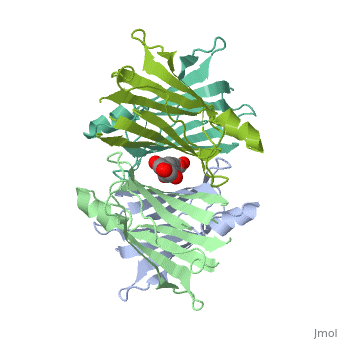
| - | <Structure load=' | + | <Structure load='3kgt' size='300' frame='true' align='right' caption='pdbcode, Insert caption here' scene='fdsfdsfsdfsdfsdfsf ' /> <scene name='48/483883/Ligand_bindings/1'>Hydrogen Bonding and Hydrophobic interactions are shown here. </scene> As you can see, there are a total of five interactions. The Hydrogen Bonds are colored blue, and the hydrophobic interactions are colored pink. The orange color is where both hydrogen bonding and hydrophobic interactions occur at the same time. |
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
==Additional Features== | ==Additional Features== | ||
| - | <Structure load=' | + | <Structure load='3kgt' size='300' frame='true' align='right' caption='pdbcode, Insert caption here' scene='Insert optional scene name here' /> |
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
==Quiz Question 1== | ==Quiz Question 1== | ||
| - | <Structure load=' | + | <Structure load='3kgt' size='300' frame='true' align='right' caption='pdbcode, Insert caption here' scene='Insert optional scene name here' /> TTR has two identical thyroxine-binding sites located at the dimer-dimer interface. Locate them. It is the blue, green or grey/red structures? <scene name='48/483883/Quiz_1/3'>Tranthyretin</scene> |
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
==Quiz Question 2== | ==Quiz Question 2== | ||
| - | <Structure load=' | + | <Structure load='3kgt' size='300' frame='true' align='right' caption='pdbcode, Insert caption here' scene='Insert optional scene name here' /> |
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
Revision as of 01:55, 13 March 2015
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Contents |
Human Transthyretin (TTR) complexed with genistein
Introduction
|
Human Transthyretin (TTR) is a gene that provides instructions for the producing of a protein called transthyretin. Transthyretin is composed of identical 127-aa sandwich subunits (shown in pink) that are produced primarily in the liver.
Overall Structure
|
Human transthyretin (TTR) is a 55 kDa homotetramer (or more precisely, a dimer of dimers) that transports thyroxine and retinol-binding protein in the blood and cerebrospinal fluid. The monomer consists of two four-stranded β-sheets, arranged in a sandwich-like tertiary structure. The intermolecular contacts formed by the dimer–dimer interface result in the formation of a spacious channel (40 A ̊ long) running along the twofold symmetry axis of the protein. The channel is about 10 A ̊ wide at the outer rim and narrows in the centre to about 4 A ̊ . This narrowing is defined by the alignment of and on the bottom of the cleft.
Binding Interactions
|
Additional Features
|
Quiz Question 1
|
Quiz Question 2
|
See Also
Credits
Introduction - name of team member
Overall Structure - name of team member
Drug Binding Site - name of team member
Additional Features - name of team member
Quiz Question 1 - name of team member
Quiz Question 2 - name of team member