We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1058
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
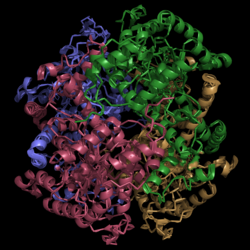
[[Image:Normal_Crystal_Structure.png|250 px|center|thumb|'''Figure 1. Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an alpha/beta barrel.]] | [[Image:Normal_Crystal_Structure.png|250 px|center|thumb|'''Figure 1. Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an alpha/beta barrel.]] | ||
Isocitrate lyase is a tetramer with 222 symmetry. Each subunit is composed of 14 alpha helices and 14 beta sheets. A unique structural feature of this enzyme is a phenomenon called "helix swapping" '''GREEN BUTTON'''. | Isocitrate lyase is a tetramer with 222 symmetry. Each subunit is composed of 14 alpha helices and 14 beta sheets. A unique structural feature of this enzyme is a phenomenon called "helix swapping" '''GREEN BUTTON'''. | ||
| - | Helix swapping is observed between two monomers to form stable dimers. The 11th and 12th helices of each monomer exchange three dimensional placement with the respective helices of the opposite monomer. Due to the 222 symmetry observed, only two dimers form than combine to form the observed tetramer. | + | Helix swapping is observed between two monomers to form stable dimers. The 11th and 12th helices of each monomer exchange three dimensional placement with the respective helices of the opposite monomer '''GREEN BUTTON'''. Due to the 222 symmetry observed, only two dimers form than combine to form the observed tetramer. |
== Function == | == Function == | ||
Revision as of 17:48, 27 March 2015
Isocitrate Lyase from Mycobacterium tuberculosis
| |||||||||||