We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1066
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
The ability for FadD13 to transport and activate fatty acids of the maximum tested length C26, lies in it being a peripheral membrane protein. FadD13 is attached to the membrane via electrostatic interactions in the N-terminal domain. Of importance in the region is the arginine rich lid-loop which serves to block the transport of fatty acids into the enzyme. Once the lid-loop is opened, fatty acids may be pulled from the membrane into a hydrophobic tunnel, which is the main structural component by which fatty acids are transported from the membrane into the cell. Negatively charged residues at the active site of FadD13 are the driving factor in the attraction of the fatty acid from the membrane through the hydrophobic tunnel of the enzyme. | The ability for FadD13 to transport and activate fatty acids of the maximum tested length C26, lies in it being a peripheral membrane protein. FadD13 is attached to the membrane via electrostatic interactions in the N-terminal domain. Of importance in the region is the arginine rich lid-loop which serves to block the transport of fatty acids into the enzyme. Once the lid-loop is opened, fatty acids may be pulled from the membrane into a hydrophobic tunnel, which is the main structural component by which fatty acids are transported from the membrane into the cell. Negatively charged residues at the active site of FadD13 are the driving factor in the attraction of the fatty acid from the membrane through the hydrophobic tunnel of the enzyme. | ||
| + | |||
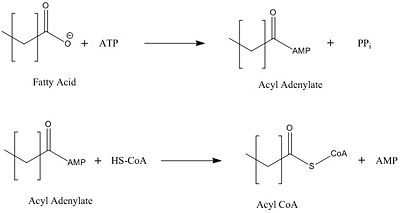
[[Image:acyl coa synthetase.jpg|400 px|center|thumb|Figure Legend]] | [[Image:acyl coa synthetase.jpg|400 px|center|thumb|Figure Legend]] | ||
= Structure = | = Structure = | ||
| Line 26: | Line 27: | ||
== Electrostatics == | == Electrostatics == | ||
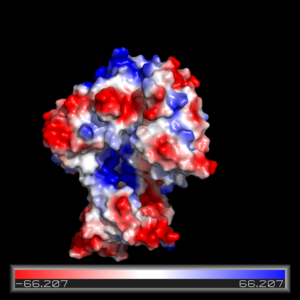
| - | [[Image:electrostatics fadD13.png| | + | [[Image:electrostatics fadD13.png|300 px|left|thumb|Figure Legend]] |
The electrostatics of FadD13 as seen in (figure...) illustrate the hydrophobic and positively charged regions that compose this protein. Experimental results from (Paper) revealed that the peripheral FadD13 attached to the membrane via positively charges interacting with the negatively charged cell membrane (image) located on the top portion of the N-terminal region. Of key importance in this N-terminal domain region attached to the membrane is an area of notable arginine rich residues, known as the arginine rich lid-loop. | The electrostatics of FadD13 as seen in (figure...) illustrate the hydrophobic and positively charged regions that compose this protein. Experimental results from (Paper) revealed that the peripheral FadD13 attached to the membrane via positively charges interacting with the negatively charged cell membrane (image) located on the top portion of the N-terminal region. Of key importance in this N-terminal domain region attached to the membrane is an area of notable arginine rich residues, known as the arginine rich lid-loop. | ||
Revision as of 01:36, 1 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Your Heading Here (maybe something like 'Structure')
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644