Sandbox Reserved 426
From Proteopedia
| Line 17: | Line 17: | ||
==Overall Structure== | ==Overall Structure== | ||
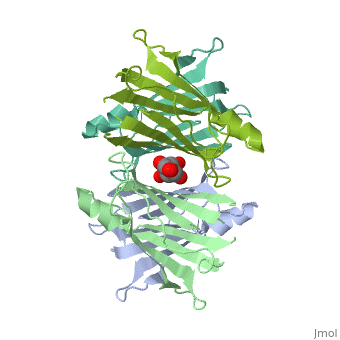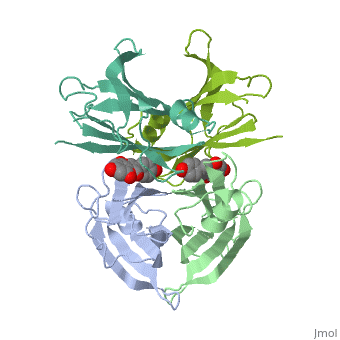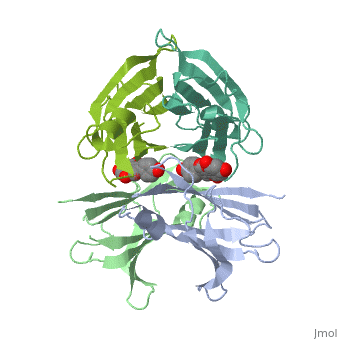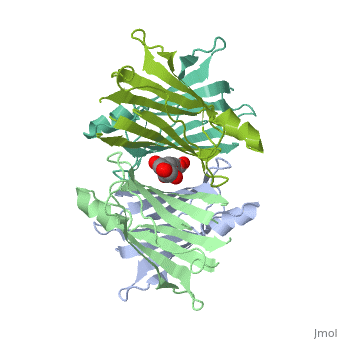
<Structure load='3kgt' size='300' frame='true' align='right' caption='Overall Structure of Human Transthyretin' scene='Sandbox_Reserved_430/> | <Structure load='3kgt' size='300' frame='true' align='right' caption='Overall Structure of Human Transthyretin' scene='Sandbox_Reserved_430/> | ||
| - | Human transthyretin (TTR) is a 55 kDa homotetramer (or more precisely, a dimer of dimers). The monomer consists of two four-stranded β-sheets, arranged in a sandwich-like tertiary structure<ref>PMID:16300401</ref>. The intermolecular contacts formed by the dimer–dimer interface result in the formation of a spacious channel (40 A ̊ long) running along the twofold symmetry axis of the protein. The <scene name='48/483883/Channel/1'>channel</scene> ( | + | Human transthyretin (TTR) is a 55 kDa homotetramer (or more precisely, a dimer of dimers). The monomer consists of two four-stranded β-sheets, arranged in a sandwich-like tertiary structure<ref>PMID:16300401</ref>. The intermolecular contacts formed by the dimer–dimer <scene name='48/483883/Interface/1'>interface</scene> result in the formation of a spacious channel (40 A ̊ long) running along the twofold symmetry axis of the protein. The <scene name='48/483883/Channel/1'>channel</scene> (with the all 4 Ser residues shown as dotted form) is about 10 A ̊ wide at the outer rim and narrows in the centre to about 4 A ̊ . This narrowing is defined by the alignment of <scene name='48/483883/Ser117a/1'>Ser117A</scene> and <scene name='48/483883/Ser117b/1'>Ser117B</scene> on the bottom of the cleft. There is a short <scene name='48/483883/Small_b-sheet/1'>β-strand</scene> (meshed for better spatial resolution), that is folded back relative to strand A via a π-turn and which is involved in the dimer–dimer contact<ref>PMID:25485123</ref>. |
<br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | <br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br><br> | ||
Revision as of 00:48, 3 April 2015
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Contents |
Human Transthyretin (TTR) complexed with genistein
Introduction
|
Encoded by Human Transthyretin gene, transthyretin (TTR) is a protein composed of identical 127-aa sandwich subunits (shown in purple). Its main function is to transport retinol and thyroxine (T4) throughout the body. Interestingly, transthyretin’s name is coming from its function: transports thyroxine and retinol. Mainly, TTR is produced by the liver, although it is also produced in smaller amounts in the choroid plexus and retinal pigment epithelium. The concentration of TTR in human plasma and cerebrospinal fluid is 0.2-0.3 mg ml-1 and 0.02 mg ml-1 respectively.
T4 is one of two major hormones produced by the thyroid gland which help control the regulation of metabolism and thus the rate at which the body uses energy. Along with two other proteins (thyroxine-binding globulin and albumin), TTR is responsible for carrying T4 in the bloodstream. In order to transport T4, four TTR proteins must bind together to form a four-protein unit (homotetramer). In addition, TTR also carries retinol (one of the major forms of vitamin A) in the blood. In this case, retinol-binding proteins (RBP) should bind to TTR (in its tetramer form).
Inappropriate folding in proteins cause a disease named amyloidosis. Amyloids (misfolded proteins) become insoluble, lose their normal function and deposit in different organs and tissues. TTR is one of the proteins that can unfold and aggregate into amyloid fibrils. TTR amyloidoses include central nervous system selective amyloidoses (CNSA), familial amyloid cardiomyopathy (FAC), familial amyloid polyneuropathy (FAP), and senile systemic amyloidosis (SSA).
Overall Structure
|
Human transthyretin (TTR) is a 55 kDa homotetramer (or more precisely, a dimer of dimers). The monomer consists of two four-stranded β-sheets, arranged in a sandwich-like tertiary structure[1]. The intermolecular contacts formed by the dimer–dimer result in the formation of a spacious channel (40 A ̊ long) running along the twofold symmetry axis of the protein. The (with the all 4 Ser residues shown as dotted form) is about 10 A ̊ wide at the outer rim and narrows in the centre to about 4 A ̊ . This narrowing is defined by the alignment of and on the bottom of the cleft. There is a short (meshed for better spatial resolution), that is folded back relative to strand A via a π-turn and which is involved in the dimer–dimer contact[2].
Binding Interactions
|
NOT FINALIZED
Outline - Hydrogen Bonds - consist of A:LYS15(3.0) A:SER117 (3.0)/ B:LYS15(3.) B:SER117 (2.7)
Hydrophobic interaction LYS15 LEU17 - LEU110 ALA108
neighboring residues / which create the binding pocket / Residues LEU17 LYS15 - LEU110 ALA108 SER117 THR119/ The tetramer forms a central hydrophobic pocket (T4 channel) with two binding sites for the ligand.
The inner sheets of the dimer–dimer (AB–CD) interface – strands A, D, G and H – form two ligand-binding site cavities, which we will refer to as sites AC and BD, respectively
The side chains from residues Lys15 and Ser117, placed at the entrance and bottom of the binding sites, respectively, make important polar contacts with the genistein hydroxyl groups (Fig. 1). Furthermore, the apolar portion of residues Leu17, Leu110, Lys15 and Ala108 contribute to genistein binding through hydrophobic contacts, as suggested by the ligand-binding analysis
Additional Features
|
This starting model is Transthyretin complexed with Genistein. The binding of substrate to Transthyretin requires four TTR proteins to be bound to each other simultaneously. This is the tetramer of TTR. As mentioned earlier, it is a homo-tetramer, this is because each of the four protein constituents are identical. The Transthyretin units are yellow, pink, green, and grey.
The binding and transport of thyroxine and retinol requires this tetramer. Retinol, the vitamin A alcohol, requires , RBP4 [3]. This is the RBP complex with . The are RBP complexed with Transthyretin. is bound with the RBP, clearly seen as non-yellow. Thyroxine bound to TTR is shown .
Quiz Question 1
|
A. An increase in binding affinity and increase in hydrophobic interactions.
B. A decrease or even complete loss of binding affinity.
C. No change in affinity, both polar and nonpolar interactions bind retinol-binding protein and transthyretin.
Quiz Question 2
|
a) How could a A108Q point mutation affect the binding of Genistein?
b) The V30M point mutation of TTR has shown to affect it's binding affinity to Genistein[4]. What Kinetic value would you expect to change for this mutant from Wild Type TTR? ( bound to Genistein, polar groups in grey, non polar groups in purple.)
See Also
Credits
Introduction - name of team member
Overall Structure - name of team member
Drug Binding Site - Sonny Nguyen, Thanh Nguyen
Additional Features - Christopher Borcoche
Quiz Question 1 - name of team member
Quiz Question 2 - Jack Caudwell
References
- ↑ Foss TR, Wiseman RL, Kelly JW. The pathway by which the tetrameric protein transthyretin dissociates. Biochemistry. 2005 Nov 29;44(47):15525-33. PMID:16300401 doi:http://dx.doi.org/10.1021/bi051608t
- ↑ Haupt M, Blakeley MP, Fisher SJ, Mason SA, Cooper JB, Mitchell EP, Forsyth VT. Binding site asymmetry in human transthyretin: insights from a joint neutron and X-ray crystallographic analysis using perdeuterated protein. IUCrJ. 2014 Oct 21;1(Pt 6):429-38. doi: 10.1107/S2052252514021113. eCollection, 2014 Nov 1. PMID:25485123 doi:http://dx.doi.org/10.1107/S2052252514021113
- ↑ Coward P, Conn M, Tang J, Xiong F, Menjares A, Reagan JD. Application of an allosteric model to describe the interactions among retinol binding protein 4, transthyretin, and small molecule retinol binding protein 4 ligands. Anal Biochem. 2009 Jan 15;384(2):312-20. doi: 10.1016/j.ab.2008.09.051. Epub 2008, Oct 12. PMID:18952041 doi:http://dx.doi.org/10.1016/j.ab.2008.09.051
- ↑ Trivella DB, Bleicher L, Palmieri Lde C, Wiggers HJ, Montanari CA, Kelly JW, Lima LM, Foguel D, Polikarpov I. Conformational differences between the wild type and V30M mutant transthyretin modulate its binding to genistein: implications to tetramer stability and ligand-binding. J Struct Biol. 2010 Jun;170(3):522-31. Epub 2010 Mar 6. PMID:20211733 doi:10.1016/j.jsb.2010.03.002