We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 427
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
==Overall Structure== | ==Overall Structure== | ||
| - | |||
| - | Copy and paste the following line where you want the scene link to appear (scroll down if needed) and edit the TextToBeDisplayed: | ||
| - | |||
| - | <scene name='48/483884/K_or_dimmer_assembly/1'>TextToBeDisplayed</scene> | ||
The Kappa opioid receptor is a dimmer composed of two identical sub units. This can be seen <scene name='48/483884/K_or_general/1'>here</scene>. The extracellular side is home to the proteins primary <scene name='48/483884/K_or_binding_general/2'>active site</scene>. These two units will span the length for the cell membrane to form the basis of the receptor molecule. The each subunit is attached to the other by the I, II and VIII alpha helices. This can be seen <scene name='48/483884/K_or_dimmer_assembly/1'>here</scene> where helices I (shown in the light blue) and helices VIII (show in the dark blue). This area will make up the basis for the intermembrane surface area. A distinguishing feature that separates the Kappa receptor from other receptors, is the large beta Hairpin,<scene name='48/483884/K_or_beta_sheet/3'>ECL2</scene>, located near the main active site of the protein. It is believed that its function is to cap the active site of the receptor. Although in general, this protein is primarily composed of alpha helices, not beta sheets (Compare <scene name='48/483884/K_or_beta_sheet/1'>beta sheet</scene> to <scene name='48/483884/K_or_alpha/1'>alpha helices </scene> here)[2]. This evidence reinforces the idea that this protein is a transmembrane protein rather than one found inside the cytosol. In general transmembrane protein are composed almost entirely of alpha helices (or beta sheets arranged in special fashion called a beta barrel), in order to have maximum stability inside the membrane [8]. Another interesting feature of the Kappa opioid receptor is the <scene name='48/483884/K_or_disulfide_bond/1'>disulfide bond </scene> formed by Cys131 and Cys210 which is conserved across all opioid receptors [2]. | The Kappa opioid receptor is a dimmer composed of two identical sub units. This can be seen <scene name='48/483884/K_or_general/1'>here</scene>. The extracellular side is home to the proteins primary <scene name='48/483884/K_or_binding_general/2'>active site</scene>. These two units will span the length for the cell membrane to form the basis of the receptor molecule. The each subunit is attached to the other by the I, II and VIII alpha helices. This can be seen <scene name='48/483884/K_or_dimmer_assembly/1'>here</scene> where helices I (shown in the light blue) and helices VIII (show in the dark blue). This area will make up the basis for the intermembrane surface area. A distinguishing feature that separates the Kappa receptor from other receptors, is the large beta Hairpin,<scene name='48/483884/K_or_beta_sheet/3'>ECL2</scene>, located near the main active site of the protein. It is believed that its function is to cap the active site of the receptor. Although in general, this protein is primarily composed of alpha helices, not beta sheets (Compare <scene name='48/483884/K_or_beta_sheet/1'>beta sheet</scene> to <scene name='48/483884/K_or_alpha/1'>alpha helices </scene> here)[2]. This evidence reinforces the idea that this protein is a transmembrane protein rather than one found inside the cytosol. In general transmembrane protein are composed almost entirely of alpha helices (or beta sheets arranged in special fashion called a beta barrel), in order to have maximum stability inside the membrane [8]. Another interesting feature of the Kappa opioid receptor is the <scene name='48/483884/K_or_disulfide_bond/1'>disulfide bond </scene> formed by Cys131 and Cys210 which is conserved across all opioid receptors [2]. | ||
| - | |||
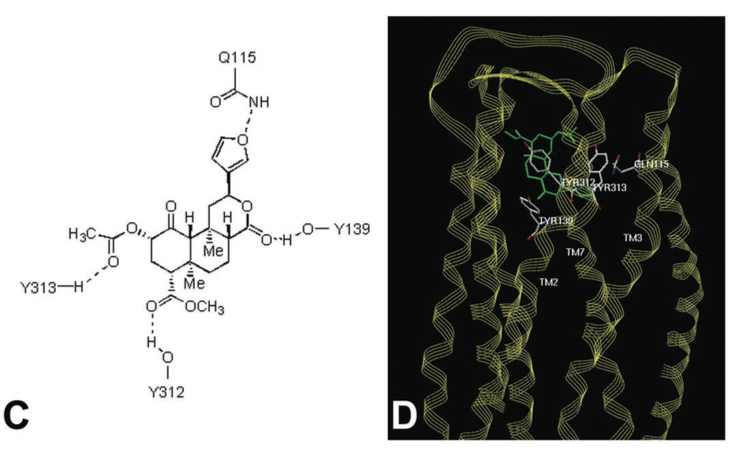
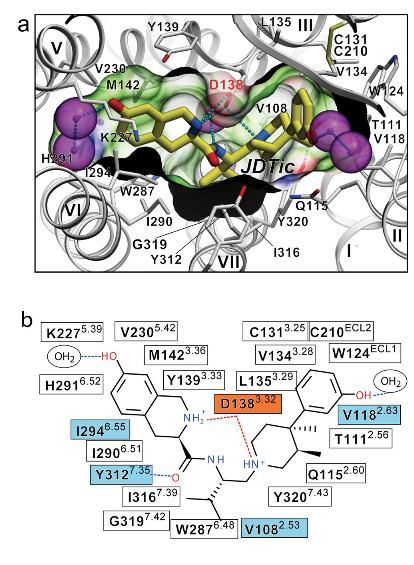
==Binding Interactions== | ==Binding Interactions== | ||
Revision as of 13:02, 3 April 2015
Ásliding right into the DMs
| This Sandbox is Reserved from January 19, 2016, through August 31, 2016 for use for Proteopedia Team Projects by the class Chemistry 423 Biochemistry for Chemists taught by Lynmarie K Thompson at University of Massachusetts Amherst, USA. This reservation includes Sandbox Reserved 425 through Sandbox Reserved 439. |
Kappa-Opioid Receptor
| |||||||||||