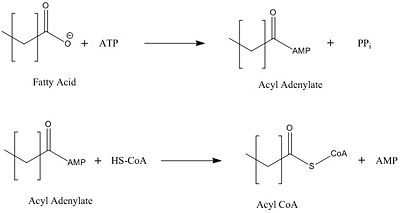
Introduction
Mycobacterium tuberculosis very-long-chain fatty acyl-CoA synthetase
Mycobacterium tuberculosis very-long-chain fatty acyl-CoA synthetase, also known as FadD13, is unique within its class in regards to its ability to house lipid substrates longer than itself. Most FadD class proteins exist as integral membrane proteins involved in lipid transport into the cell. FadD13 is unique structurally in that it exists as a peripheral protein on the inside of the cell membrane. This feature is key in the mechanistic basis for FadD13's transport of fatty acids of length C24-C26.[1]
FadD13 may be important in the virulence of Mycobacterium tuberculosis, the etiological agent of tuberculosis, and has emerged as possible target for novel therapeutic agents.[2]
Mechanism
General mechanism for the activation of fatty acids
Structural basis for housing lipid substrates longer than the enzyme
The ability for FadD13 to transport and activate fatty acids of the maximum tested length C26, lies in it being a peripheral membrane protein. FadD13 is attached to the membrane via electrostatic interactions in the N-terminal domain. Of importance in the region is the arginine rich lid-loop which serves to block the transport of fatty acids into the enzyme. Once the lid-loop is opened, fatty acids may be pulled from the membrane into a hydrophobic tunnel, which is the main structural component by which fatty acids are transported from the membrane into the cell. Negatively charged residues at the active site of FadD13 are the driving factor in the attraction of the fatty acid from the membrane through the hydrophobic tunnel of the enzyme.
Structure
General overview
This is the
FadD13 is composed of 503 amino acid residues divided into three main regions: The (residues 1-395) and (residues 402-503) which are connected via a flexible linker (residues 396-401).[1]
Electrostatics
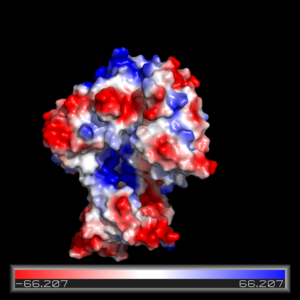
Figure Legend. Pmyol depiction of electrostatic interaction of FadD13.
The electrostatics of FadD13 as seen in (figure...) illustrate the hydrophobic and positively charged regions that compose this protein. Experimental results from (Paper) revealed that the peripheral FadD13 attached to the membrane via positively charges interacting with the negatively charged cell membrane (image) located on the top portion of the N-terminal region. Of key importance in this N-terminal domain region attached to the membrane is an area of notable arginine rich residues, known as the arginine rich lid-loop.
Arginine Rich Lid-loop
The functions to block entry of fatty acids into the hydrophobic tunnel of FadD13.
Hydrophobic Tunnel
The hydrophobic tunnel of FadD13 is essential to the transport and accommodation of very long fatty acids from the membrane into the cell. This tunnel runs through the middle of FadD13 from the arginine rich lid loop to the ATP binding site and is situated between the and alpha helices α8-α9 and parallel beta sheet β9- β14.[1]
Active Site
The active site on FadD13 is composed of two conserved regions, one of which serves as the binding site for ATP and the other for CoA. The
Disease
Mycobacterium tuberculosis is the causative agent involved in the disease tuberculosis.
Relevance
Inhibitors
Future Research
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.