User:Braden Sciarra/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== Structure == | == Structure == | ||
| + | |||
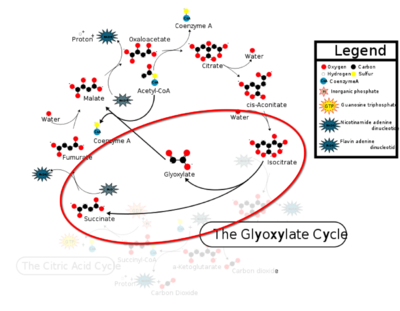
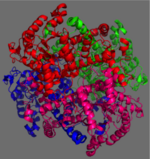
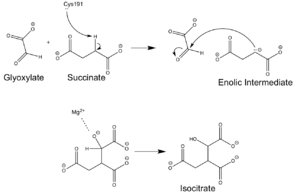
| + | The ICL homotetramer possesses 222 symmetry, with an axis of rotation at x-axis, y-axis, and z-axis of the enzyme. Two individual subunits off ICL are held together by a characteristic <scene name='69/697526/Helix_swapping/3'>Helix Swapping</scene> between three alpha helices formed by residues 370-384, 349-367, and 399-409 on neighboring monomers. The interlocking mechanism created by these helices provide additional strength to hold the two monomeric subunits together, allowing ICL to essentially be composed of two dimerized subunits. This interaction will bury approximately 18% of the surface of each subunit, and will help to shield the interior binding site from hydration. | ||
<scene name='69/697526/Helix_swapping/3'>Helix Swapping</scene> | <scene name='69/697526/Helix_swapping/3'>Helix Swapping</scene> | ||
Revision as of 22:40, 7 April 2015
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||