User:Braden Sciarra/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
===Catalytic Loop=== | ===Catalytic Loop=== | ||
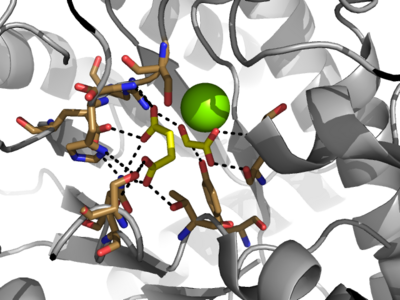
| - | + | [[Image:Openvsclosed.png|400 px|left|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL]] | |
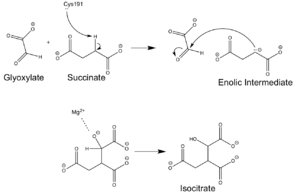
The <scene name='69/697526/Catalytic_loop/1'>Catalytic Loop</scene> of the Isocitrate Lyase enzyme is composed of residues 185-196, and can exist in both the open and closed conformation. In the open conformation, the catalytic loop is oriented such that the catalytic CYS191 residue is located far from the active site, allowing for solvent accessibility and substrate binding. Upon substrate binding, the catalytic loop adopts a closed conformation, moving between ten and fifteen angstroms. This closed conformation will cause the binding site to become inaccessible to the solvent. The loop closure is triggered by the movement of the Mg2+ ion that occurs upon binding of the succinate. This movement of the Mg2+ ion results in electrostatic interactions at LYS189, causing the loop to close. | The <scene name='69/697526/Catalytic_loop/1'>Catalytic Loop</scene> of the Isocitrate Lyase enzyme is composed of residues 185-196, and can exist in both the open and closed conformation. In the open conformation, the catalytic loop is oriented such that the catalytic CYS191 residue is located far from the active site, allowing for solvent accessibility and substrate binding. Upon substrate binding, the catalytic loop adopts a closed conformation, moving between ten and fifteen angstroms. This closed conformation will cause the binding site to become inaccessible to the solvent. The loop closure is triggered by the movement of the Mg2+ ion that occurs upon binding of the succinate. This movement of the Mg2+ ion results in electrostatic interactions at LYS189, causing the loop to close. | ||
Revision as of 23:14, 7 April 2015
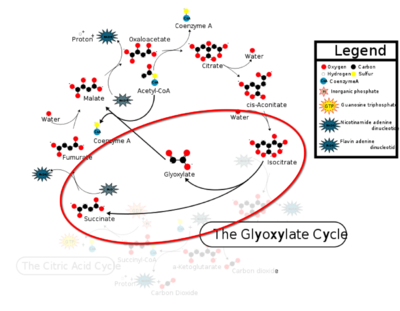
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||