User:Braden Sciarra/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Active Site == | == Active Site == | ||
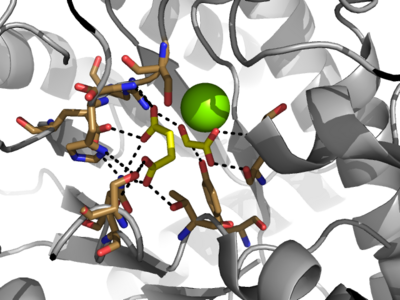
[[Image:Serine_with_active_site.png|400 px|right|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL]] | [[Image:Serine_with_active_site.png|400 px|right|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL]] | ||
| - | The <scene name='69/697526/Binding_pocket/1'>Active Site</scene> of isocitrate lyase lies near the C-terminal ends of the Beta-strands of the active site. The glyoxylate substrate is held into place by a network of hydrogen bonds with Ser 91, Gly 92, Trp 93, and Arg 228. The magnesium ion serves to stabilize the partial negative charge placed on the carbonyl oxygens of the glyoxylate. The other substrate, succinate, contains two carboxyl groups and possesses a similar network of hydrogen bonding that holds the ligand in place within the active site. One carboxylate group is hydrogen bound to Asn 313, Glu 295, Arg 228, and Gly 192. The second carboxylate is hydrogen bound to Thr 347, Asn 313, Ser 315, Ser 317, and His 193. The ordered hydrogen bonding within the active site orients the succinate molecule such that the | + | The <scene name='69/697526/Binding_pocket/1'>Active Site</scene> of isocitrate lyase lies near the C-terminal ends of the Beta-strands of the active site. The glyoxylate substrate is held into place by a network of hydrogen bonds with Ser 91, Gly 92, Trp 93, and Arg 228. The magnesium ion serves to stabilize the partial negative charge placed on the carbonyl oxygens of the glyoxylate. The other substrate, succinate, contains two carboxyl groups and possesses a similar network of hydrogen bonding that holds the ligand in place within the active site. One carboxylate group is hydrogen bound to Asn 313, Glu 295, Arg 228, and Gly 192. The second carboxylate is hydrogen bound to Thr 347, Asn 313, Ser 315, Ser 317, and His 193. The ordered hydrogen bonding within the active site orients the succinate molecule such that the alpha carbon is only 3.2 angstroms away from the deprotonated thiol group of Cys 191. |
===Catalytic Loop=== | ===Catalytic Loop=== | ||
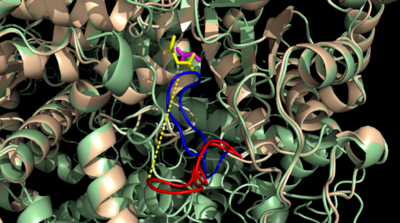
[[Image:Openvsclosed..png|400 px|left|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL]] | [[Image:Openvsclosed..png|400 px|left|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL]] | ||
The <scene name='69/697526/Catalytic_loop/1'>Catalytic Loop</scene> of the Isocitrate Lyase enzyme is composed of residues 185-196, and can exist in both the open and closed conformation. In the open conformation, the catalytic loop is oriented such that the catalytic CYS191 residue is located far from the active site, allowing for solvent accessibility and substrate binding. Upon substrate binding, the catalytic loop adopts a closed conformation, moving between ten and fifteen angstroms. This closed conformation will cause the binding site to become inaccessible to the solvent. The loop closure is triggered by the movement of the Mg2+ ion that occurs upon binding of the succinate. This movement of the Mg2+ ion results in electrostatic interactions at LYS189, causing the loop to close. | The <scene name='69/697526/Catalytic_loop/1'>Catalytic Loop</scene> of the Isocitrate Lyase enzyme is composed of residues 185-196, and can exist in both the open and closed conformation. In the open conformation, the catalytic loop is oriented such that the catalytic CYS191 residue is located far from the active site, allowing for solvent accessibility and substrate binding. Upon substrate binding, the catalytic loop adopts a closed conformation, moving between ten and fifteen angstroms. This closed conformation will cause the binding site to become inaccessible to the solvent. The loop closure is triggered by the movement of the Mg2+ ion that occurs upon binding of the succinate. This movement of the Mg2+ ion results in electrostatic interactions at LYS189, causing the loop to close. | ||
| - | |||
== Mechanism == | == Mechanism == | ||
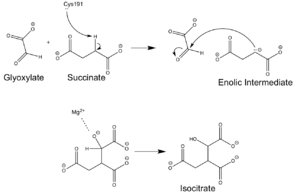
| - | + | [[Image:ICL Mechanism.png|300 px|right|thumb|Chemical Mechanism of Isocitrate Lyase]] | |
| - | [[Image:ICL Mechanism.png|300 px| | + | Isocitrate lyase catalyzes a reversible aldol condensation, converting isocitrate to glyoxylate and succinate via the breaking of a C-C bond. Within the active site of ICL the HIS193 residue deprotonates the CYS191 residue of the active site in order to increase its basicity. The Cys 191 residue then deprotonates the alpha carbon adjacent to one of the carbonyl groups of succinate, thus forming the enolic intermediate. The negatively charged alpha carbon atom of the enolic intermediate acts as a nucleophile that attacks the carbonyl carbon of the aldehyde of glyoxylate. The nucleophilic attack will place a negative charge on the oxygen atom oxygen at the former carbonyl oxygen of the aldehyde, which will be stabilized by positive charges of the Mg2+ ion, Arg 228 and His 180. The protonation of this species will yield the final product. It is important to note that this reaction is entirely reversible; the breakdown of isocitrate into glyoxylate and succinate occurs using a similar mechanism. |
| - | + | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 23:24, 7 April 2015
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||