User:Braden Sciarra/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 17: | Line 17: | ||
==Mechanism== | ==Mechanism== | ||
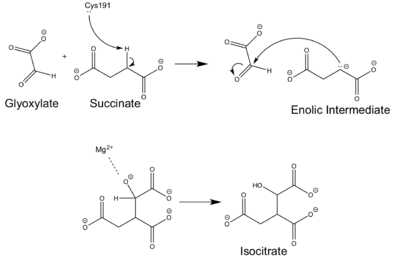
| - | [[Image:ICL Mechanism.png|400 px|right|thumb|Chemical Mechanism of Isocitrate Lyase]] | + | [[Image:ICL Mechanism.png|400 px|right|thumb|Figure 4: Chemical Mechanism of Isocitrate Lyase]] |
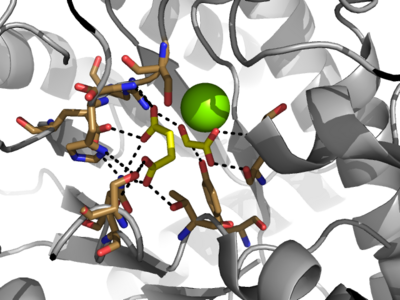
| - | Isocitrate lyase catalyzes a reversible aldol condensation, converting isocitrate to glyoxylate and succinate via the breaking of a C-C bond. Within the active site of ICL the HIS193 residue deprotonates the CYS191 residue of the active site in order to increase its basicity<ref name="ICL">PMID:10932251</ref>. The Cys 191 residue then deprotonates the alpha carbon adjacent to one of the carbonyl groups of succinate, thus forming the enolic intermediate. The negatively charged alpha carbon atom of the enolic intermediate acts as a nucleophile that attacks the carbonyl carbon of the aldehyde of glyoxylate. The nucleophilic attack will place a negative charge on the oxygen atom oxygen at the former carbonyl oxygen of the aldehyde, which will be stabilized by positive charges of the Mg2+ ion, | + | Isocitrate lyase catalyzes a reversible aldol condensation, converting isocitrate to glyoxylate and succinate via the breaking of a C-C bond. Within the active site of ICL the HIS193 residue deprotonates the CYS191 residue of the active site in order to increase its basicity<ref name="ICL">PMID:10932251</ref>. The Cys 191 residue then deprotonates the alpha carbon adjacent to one of the carbonyl groups of succinate, thus forming the enolic intermediate. The negatively charged alpha carbon atom of the enolic intermediate acts as a nucleophile that attacks the carbonyl carbon of the aldehyde of glyoxylate. The nucleophilic attack will place a negative charge on the oxygen atom oxygen at the former carbonyl oxygen of the aldehyde, which will be stabilized by positive charges of the Mg2+ ion, ARG228 and HIS180<ref name="ICL">PMID:10932251</ref>. The protonation of this species will yield the final product. It is important to note that this reaction is entirely reversible; the breakdown of isocitrate into glyoxylate and succinate occurs using a similar mechanism. |
==Elucidation of ICL Structure Using Inhibitors== | ==Elucidation of ICL Structure Using Inhibitors== | ||
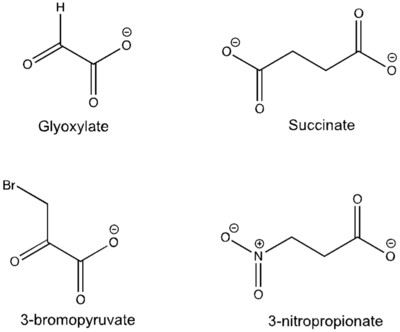
| - | [[Image:Inhibitors57.png|400 px|right|thumb|Figure | + | [[Image:Inhibitors57.png|400 px|right|thumb|Figure 5: Substrate inhibitors of Isocitrate Lyase]] |
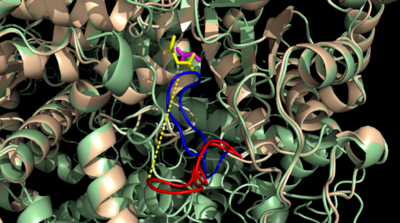
| - | The two inhibitors used to elucidate the structure of ICL were 3-nitropropionate and 3-bromopyruvate. The 3-nitropropionate was used to mimic the succinate, while the 3-bromopyruvate is used to mimic the glyoxylate. These two inhibitors have also been shown to be good inhibitors of isocitrate lyase in ''M. avium'' indicating that their inhibitory capacity is conserved across multiple species. A mutant isocitrate lyase C191S, in conjunction with the aforementioned substrate mimics, was used to elucidate the first high resolution crystal structure of ICL. Dehalogenated 3-nitropropionate works to inhibit isocitrate lyase by forming a covalent bond with the | + | The two inhibitors used to elucidate the structure of ICL were 3-nitropropionate and 3-bromopyruvate. The 3-nitropropionate was used to mimic the succinate, while the 3-bromopyruvate is used to mimic the glyoxylate. These two inhibitors have also been shown to be good inhibitors of isocitrate lyase in ''M. avium'' indicating that their inhibitory capacity is conserved across multiple species. A mutant isocitrate lyase C191S, in conjunction with the aforementioned substrate mimics, was used to elucidate the first high resolution crystal structure of ICL. Dehalogenated 3-nitropropionate works to inhibit isocitrate lyase by forming a covalent bond with the SER191 residue in the active site. This 3-nitropropionate occupies the same site that the succinate would occupy. The C191S mutant adopts a conformation almost identical to the CYS191 residue in the wild type indicating that this is an accurate depiction of the conformation. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 00:01, 8 April 2015
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||