User:Braden Sciarra/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
== Structure == | == Structure == | ||
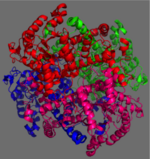
[[Image:homotetramer.png|150 px|left|thumb|Figure 2: C2 Symmetry of the homotetramer isocitrate lyase]] | [[Image:homotetramer.png|150 px|left|thumb|Figure 2: C2 Symmetry of the homotetramer isocitrate lyase]] | ||
| - | The ICL homotetramer possesses C2 symmetry, with an axis of rotation at x-axis, y-axis, and z-axis of the enzyme. Two individual subunits off ICL are held together by a characteristic <scene name='69/697526/Helix_swapping/3'>Helix Swapping</scene> between three alpha helices formed by residues 370-384, 349-367, and 399-409 on neighboring monomers<ref name="ICL"</ref>. The interlocking mechanism created by these helices provides additional strength to hold the two monomeric subunits together, allowing ICL to essentially be composed of two dimerized subunits. This interaction will bury approximately 18% of the surface of each subunit, and will help to shield the interior binding site from hydration. | + | The ICL homotetramer possesses C2 symmetry, with an axis of rotation at x-axis, y-axis, and z-axis of the enzyme. Two individual subunits off ICL are held together by a characteristic <scene name='69/697526/Helix_swapping/3'>Helix Swapping</scene> between three alpha helices formed by residues 370-384, 349-367, and 399-409 on neighboring monomers<ref name="ICL">PMID:10932251</ref>. The interlocking mechanism created by these helices provides additional strength to hold the two monomeric subunits together, allowing ICL to essentially be composed of two dimerized subunits. This interaction will bury approximately 18% of the surface of each subunit, and will help to shield the interior binding site from hydration. |
== Active Site == | == Active Site == | ||
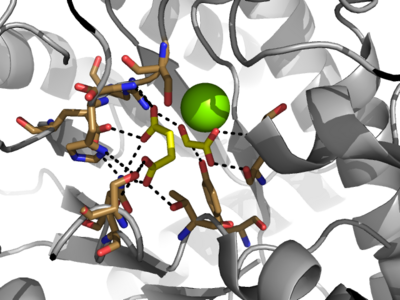
[[Image:Serine_with_active_site.png|400 px|right|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL]] | [[Image:Serine_with_active_site.png|400 px|right|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL]] | ||
| - | The <scene name='69/697526/Binding_pocket/1'>Active Site</scene> of isocitrate lyase lies near the C-terminal ends of the Beta-strands of the active site. The glyoxylate substrate is held into place by a network of hydrogen bonds with Ser 91, Gly 92, Trp 93, and Arg 228<ref name="ICL"</ref>. The magnesium ion serves to stabilize the partial negative charge placed on the carbonyl oxygens of the glyoxylate. The other substrate, succinate, contains two carboxyl groups and possesses a similar network of hydrogen bonding that holds the ligand in place within the active site. One carboxylate group is hydrogen bound to Asn 313, Glu 295, Arg 228, and Gly 192. The second carboxylate is hydrogen bound to Thr 347, Asn 313, Ser 315, Ser 317, and His 193<ref name="ICL"</ref>. The ordered hydrogen bonding within the active site orients the succinate molecule such that the alpha carbon is only 3.2 angstroms away from the deprotonated thiol group of Cys 191<ref name="ICL"</ref>. | + | The <scene name='69/697526/Binding_pocket/1'>Active Site</scene> of isocitrate lyase lies near the C-terminal ends of the Beta-strands of the active site. The glyoxylate substrate is held into place by a network of hydrogen bonds with Ser 91, Gly 92, Trp 93, and Arg 228<ref name="ICL">PMID:10932251</ref>>. The magnesium ion serves to stabilize the partial negative charge placed on the carbonyl oxygens of the glyoxylate. The other substrate, succinate, contains two carboxyl groups and possesses a similar network of hydrogen bonding that holds the ligand in place within the active site. One carboxylate group is hydrogen bound to Asn 313, Glu 295, Arg 228, and Gly 192. The second carboxylate is hydrogen bound to Thr 347, Asn 313, Ser 315, Ser 317, and His 193<ref name="ICL">PMID:10932251</ref>. The ordered hydrogen bonding within the active site orients the succinate molecule such that the alpha carbon is only 3.2 angstroms away from the deprotonated thiol group of Cys 191<ref name="ICL">PMID:10932251</ref>. |
===Catalytic Loop=== | ===Catalytic Loop=== | ||
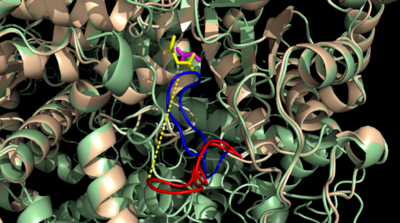
[[Image:Openvsclosed..png|400 px|left|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL]] | [[Image:Openvsclosed..png|400 px|left|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL]] | ||
| - | The <scene name='69/697526/Catalytic_loop/1'>Catalytic Loop</scene> of the Isocitrate Lyase enzyme is composed of residues 185-196, and can exist in both the open and closed conformation. In the open conformation, the catalytic loop is oriented such that the catalytic CYS191 residue is located far from the active site, allowing for solvent accessibility and substrate binding. Upon substrate binding, the catalytic loop adopts a closed conformation, moving between ten and fifteen angstroms<ref name="ICL"</ref>. This closed conformation will cause the binding site to become inaccessible to the solvent. The loop closure is triggered by the movement of the Mg2+ ion that occurs upon binding of the succinate. This movement of the Mg2+ ion results in electrostatic interactions at LYS189, causing the loop to close. | + | The <scene name='69/697526/Catalytic_loop/1'>Catalytic Loop</scene> of the Isocitrate Lyase enzyme is composed of residues 185-196, and can exist in both the open and closed conformation. In the open conformation, the catalytic loop is oriented such that the catalytic CYS191 residue is located far from the active site, allowing for solvent accessibility and substrate binding. Upon substrate binding, the catalytic loop adopts a closed conformation, moving between ten and fifteen angstroms<ref name="ICL">PMID:10932251</ref>. This closed conformation will cause the binding site to become inaccessible to the solvent. The loop closure is triggered by the movement of the Mg2+ ion that occurs upon binding of the succinate. This movement of the Mg2+ ion results in electrostatic interactions at LYS189, causing the loop to close. |
==Mechanism== | ==Mechanism== | ||
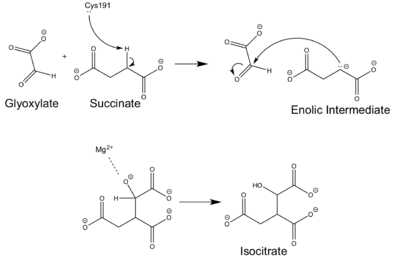
[[Image:ICL Mechanism.png|400 px|right|thumb|Figure 4: Chemical Mechanism of Isocitrate Lyase]] | [[Image:ICL Mechanism.png|400 px|right|thumb|Figure 4: Chemical Mechanism of Isocitrate Lyase]] | ||
| - | Isocitrate lyase catalyzes a reversible aldol condensation, converting isocitrate to glyoxylate and succinate via the breaking of a C-C bond. Within the active site of ICL the HIS193 residue deprotonates the CYS191 residue of the active site in order to increase its basicity<ref name="ICL"</ref>. The Cys 191 residue then deprotonates the alpha carbon adjacent to one of the carbonyl groups of succinate, thus forming the enolic intermediate. The negatively charged alpha carbon atom of the enolic intermediate acts as a nucleophile that attacks the carbonyl carbon of the aldehyde of glyoxylate. The nucleophilic attack will place a negative charge on the oxygen atom oxygen at the former carbonyl oxygen of the aldehyde, which will be stabilized by positive charges of the Mg2+ ion, ARG228 and HIS180<ref name="ICL"</ref>. The protonation of this species will yield the final product. It is important to note that this reaction is entirely reversible; the breakdown of isocitrate into glyoxylate and succinate occurs using a similar mechanism. | + | Isocitrate lyase catalyzes a reversible aldol condensation, converting isocitrate to glyoxylate and succinate via the breaking of a C-C bond. Within the active site of ICL the HIS193 residue deprotonates the CYS191 residue of the active site in order to increase its basicity<ref name="ICL"</ref>. The Cys 191 residue then deprotonates the alpha carbon adjacent to one of the carbonyl groups of succinate, thus forming the enolic intermediate. The negatively charged alpha carbon atom of the enolic intermediate acts as a nucleophile that attacks the carbonyl carbon of the aldehyde of glyoxylate. The nucleophilic attack will place a negative charge on the oxygen atom oxygen at the former carbonyl oxygen of the aldehyde, which will be stabilized by positive charges of the Mg2+ ion, ARG228 and HIS180<ref name="ICL">PMID:10932251</ref>. The protonation of this species will yield the final product. It is important to note that this reaction is entirely reversible; the breakdown of isocitrate into glyoxylate and succinate occurs using a similar mechanism. |
==Elucidation of ICL Structure Using Inhibitors== | ==Elucidation of ICL Structure Using Inhibitors== | ||
Revision as of 00:08, 8 April 2015
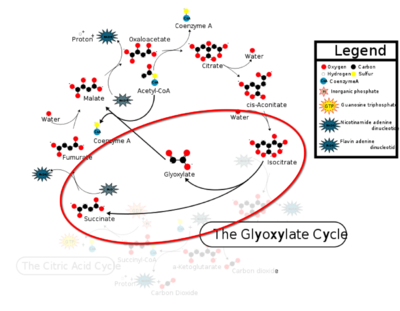
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Sharma V, Sharma S, Hoener zu Bentrup K, McKinney JD, Russell DG, Jacobs WR Jr, Sacchettini JC. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat Struct Biol. 2000 Aug;7(8):663-8. PMID:10932251 doi:10.1038/77964
- ↑ . The Cys 191 residue then deprotonates the alpha carbon adjacent to one of the carbonyl groups of succinate, thus forming the enolic intermediate. The negatively charged alpha carbon atom of the enolic intermediate acts as a nucleophile that attacks the carbonyl carbon of the aldehyde of glyoxylate. The nucleophilic attack will place a negative charge on the oxygen atom oxygen at the former carbonyl oxygen of the aldehyde, which will be stabilized by positive charges of the Mg2+ ion, ARG228 and HIS180<ref>PMID:10932251</li></ol></ref>