Sandbox Reserved 1074
From Proteopedia
(Difference between revisions)
| Line 26: | Line 26: | ||
=== '''Fatty Acyl Binding Crevice''' === | === '''Fatty Acyl Binding Crevice''' === | ||
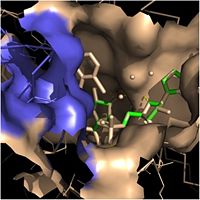
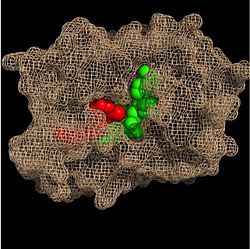
| - | Within the fatty acyl binding crevice, the NADH substrate sits on the top shelf of the Rossmann fold, and the fatty acyl substrate sits on top of the NADH substrate. | + | Within the fatty acyl binding crevice, the NADH substrate sits on the top shelf of the Rossmann fold, and the fatty acyl substrate sits on top of the NADH substrate. Due to its position within the crevice, the ''trans'' double bond of the fatty acyl substrate is found near the closed end of the crevice and is located directly adjacent to the nicotinamide ring of [http://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NADH]. [[Image:Binding Pocket - Mesh.jpg|thumb|250px|right|Substrate Binding Pocket (NADH in green; fatty acyl substrate in red)]]Both of these substrates are held in place through interactions with the side chains of <scene name='69/694241/Sbl_hydrophobic/1'>hydrophobic</scene> (purple) residues. The majority of these <scene name='69/694241/Hydrophobic_residues/1'>hydrophobic</scene> residues anchoring the substrates are found within the substrate binding loop itself, including Ala-198, Met-199, Ala-201, Ile-202, Leu-207, Ile-215, and Leu-218. Additional hydrophobic amino acids that are not a part of the substrate binding loop also play a role in positioning and stabilizing the fatty acyl chain in the crevice. Studies have found that the fatty acyl substrate adopts a u-shaped conformation to facilitate binding. |
| - | <scene name='69/694241/Nadh_fatty_acyl_substrate/2'>NADH and fatty acyl binding substrate</scene> | ||
| Line 44: | Line 43: | ||
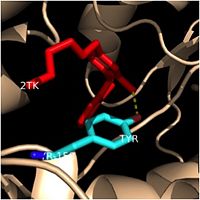
=== '''Hydrogen Bonding Interactions''' === | === '''Hydrogen Bonding Interactions''' === | ||
| - | |||
| - | |||
| - | |||
| - | |||
Revision as of 01:45, 8 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Enoyl-ACP Reductase InhA from Mycobacterium tuberculosis
| |||||||||||