Sandbox Reserved 1074
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
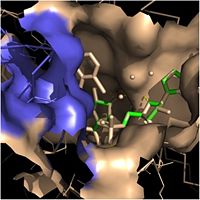
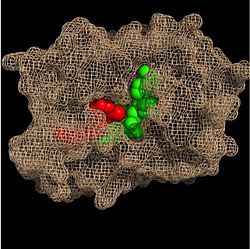
Within the fatty acyl binding crevice, the NADH substrate sits on the top shelf of the Rossmann fold, and the fatty acyl substrate sits on top of the NADH substrate. Due to its position within the crevice, the ''trans'' double bond of the fatty acyl substrate is found near the closed end of the crevice and is located directly adjacent to the nicotinamide ring of [http://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NADH]. [[Image:Binding Pocket - Mesh.jpg|thumb|250px|right|Substrate Binding Pocket (NADH in green; fatty acyl substrate in red)]] These two molecules interact with each other via hydrogen bonding. The first hydrogen bond occurs between the phosphate oxygen of NADH and the amide nitrogen of the N-acetylcysteamine portion of the fatty acyl substrate. The second hydrogen bond occurs between the 2'-hydroxyl of the nicotinamide ribose of NADH and the thioester carbonyl oxygen of the fatty acyl substrate. | Within the fatty acyl binding crevice, the NADH substrate sits on the top shelf of the Rossmann fold, and the fatty acyl substrate sits on top of the NADH substrate. Due to its position within the crevice, the ''trans'' double bond of the fatty acyl substrate is found near the closed end of the crevice and is located directly adjacent to the nicotinamide ring of [http://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NADH]. [[Image:Binding Pocket - Mesh.jpg|thumb|250px|right|Substrate Binding Pocket (NADH in green; fatty acyl substrate in red)]] These two molecules interact with each other via hydrogen bonding. The first hydrogen bond occurs between the phosphate oxygen of NADH and the amide nitrogen of the N-acetylcysteamine portion of the fatty acyl substrate. The second hydrogen bond occurs between the 2'-hydroxyl of the nicotinamide ribose of NADH and the thioester carbonyl oxygen of the fatty acyl substrate. | ||
| + | |||
==='''Substrate Binding Loop Flexibility''' === | ==='''Substrate Binding Loop Flexibility''' === | ||
| Line 32: | Line 33: | ||
In addition to the hydrogen bonding that occurs between the NADH and fatty acyl substrate within the crevice, each of these molecules is also held in place within the crevice through interactions with the side chains of surrounding <scene name='69/694241/Sbl_hydrophobic/1'>hydrophobic</scene> (purple) residues. The majority of these <scene name='69/694241/Hydrophobic_residues/1'>hydrophobic</scene> residues anchoring the substrates are found within the substrate binding loop itself, including Ala-198, Met-199, Ala-201, Ile-202, Leu-207, Ile-215, and Leu-218. Additional hydrophobic amino acids that are not a part of the substrate binding loop also play a role in positioning and stabilizing the fatty acyl chain in the crevice. Studies have found that the fatty acyl substrate adopts a u-shaped conformation to facilitate binding. As the fatty acyl substrate binds in the crevice, the substrate binding loop shifts outward toward the solvent, and the [[Tyr-158]] residue is rotated to facilitate the binding of fatty acyl chains of 16 carbons or greater. During this conformational change upon substrate binding, no hydrogen bonds are broken, which supports the flexibility of the substrate binding loop. It is likely that this flexibility of the substrate binding loop provides increased freedom for the active site to accept fatty acyl substrates of varying carbon chain lengths. | In addition to the hydrogen bonding that occurs between the NADH and fatty acyl substrate within the crevice, each of these molecules is also held in place within the crevice through interactions with the side chains of surrounding <scene name='69/694241/Sbl_hydrophobic/1'>hydrophobic</scene> (purple) residues. The majority of these <scene name='69/694241/Hydrophobic_residues/1'>hydrophobic</scene> residues anchoring the substrates are found within the substrate binding loop itself, including Ala-198, Met-199, Ala-201, Ile-202, Leu-207, Ile-215, and Leu-218. Additional hydrophobic amino acids that are not a part of the substrate binding loop also play a role in positioning and stabilizing the fatty acyl chain in the crevice. Studies have found that the fatty acyl substrate adopts a u-shaped conformation to facilitate binding. As the fatty acyl substrate binds in the crevice, the substrate binding loop shifts outward toward the solvent, and the [[Tyr-158]] residue is rotated to facilitate the binding of fatty acyl chains of 16 carbons or greater. During this conformational change upon substrate binding, no hydrogen bonds are broken, which supports the flexibility of the substrate binding loop. It is likely that this flexibility of the substrate binding loop provides increased freedom for the active site to accept fatty acyl substrates of varying carbon chain lengths. | ||
| - | + | ||
| + | ==='''Importance of Tyr-158'''=== | ||
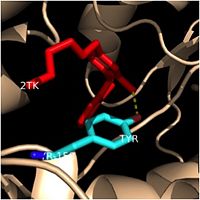
One example of a hydrophobic amino acid that is not a part of the substrate binding loop yet interacts with the fatty acyl substrate is [[Tyr-158]]. [[Image:Tyr-158.jpg|thumb|200px|left|Tyr-158 (turquoise) hydrogen bonding to the fatty acyl substrate, 2TK (red)]] This amino acid is conserved in other enoyl-ACP reductases in both bacteria and plants, so it likely plays an essential role in the function of these specific enzymes. Studies have shown that Tyr-158 forms the only direct hydrogen bond that exists between the InhA protein and the fatty acyl substrate. This hydrogen bond occurs between the hydroxyl oxygen on the side chain of Tyr-158 and the thioester carbonyl oxygen of the fatty acyl substrate. As a result of the highly conserved nature and the specific hydrogen bonding capabilities of this amino acid, it is likely that Tyr-158 plays an important role in fatty acyl substrate binding in enoyl-ACP reductases. | One example of a hydrophobic amino acid that is not a part of the substrate binding loop yet interacts with the fatty acyl substrate is [[Tyr-158]]. [[Image:Tyr-158.jpg|thumb|200px|left|Tyr-158 (turquoise) hydrogen bonding to the fatty acyl substrate, 2TK (red)]] This amino acid is conserved in other enoyl-ACP reductases in both bacteria and plants, so it likely plays an essential role in the function of these specific enzymes. Studies have shown that Tyr-158 forms the only direct hydrogen bond that exists between the InhA protein and the fatty acyl substrate. This hydrogen bond occurs between the hydroxyl oxygen on the side chain of Tyr-158 and the thioester carbonyl oxygen of the fatty acyl substrate. As a result of the highly conserved nature and the specific hydrogen bonding capabilities of this amino acid, it is likely that Tyr-158 plays an important role in fatty acyl substrate binding in enoyl-ACP reductases. | ||
| Line 39: | Line 41: | ||
=== '''Catalytic Triad''' === | === '''Catalytic Triad''' === | ||
| - | <scene name='69/694241/Catalytic_triad/1'>catalytic triad</scene> | + | Structural studies have shown that InhA possesses the <scene name='69/694241/Catalytic_triad/1'> Phe-Tyr-Lys catalytic triad</scene>, composed of Phe-149, Tyr-158, and Lys-165. This catalytic triad of InhA is analogous to the classic Ser-Tyr-Lys catalytic triad of the SDR (Short-chain Dehydrogenase Reductase) family in terms of position and function within the enzyme. As discussed previously, the likely role of Tyr-158 is to position the fatty acyl substrate within the fatty acyl binding crevice. Second, the side chain of the Lys-165 residue in InhA functions similarly to the catalytic Lys residues in other SDR enzymes by interacting with the 3'-hydroxyl of the nicotinamide ring of NADH to hold this cofactor in place within the fatty acyl binding crevice. Finally, the exact role of the catalytic Phe-149 residue in InhA is unknown. It has been hypothesized to play a role in helping the fatty acyl substrate adopt its desired u-shape prior to binding. However, it is more widely believed that this catalytic residue merely distinguishes InhA as a dehydrogenase. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Revision as of 02:43, 8 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Enoyl-ACP Reductase InhA from Mycobacterium tuberculosis
| |||||||||||