Sandbox Reserved 1074
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
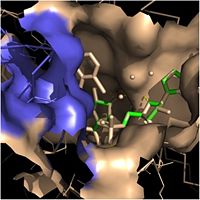
Crystal structures of InhA reveal a <scene name='69/694241/Homotetramer_subunits_labeled/1'>homotetramer</scene> (each subunit featured with a different color) in aqueous solution with separate ligand binding sites in each subunit. Each <scene name='69/694241/Monomer_subunit_no_ligands/1'>monomer</scene> subunit is composed of 289 residues and features a typical [http://en.wikipedia.org/wiki/Rossmann_fold Rossmann fold] containing a single NADH binding site. The <scene name='69/694241/Secondary_structure_black/1'>secondary structure</scene> of InhA is made up of several alpha helices (pink), beta sheets (gold), and beta turns (white). This enzyme also features a fatty acyl binding crevice that accommodates the long-chain fatty acyl substrate needed to synthesize mycolic acid precursors. The <scene name='69/694241/Helix6_helix7_updated/1'>alpha-6 and alpha-7 helices</scene> of the InhA form one side of the fatty acyl binding crevice, referred to as the <scene name='69/694241/Monomer_subunit_196_219/1'> substrate binding loop</scene> (residues 196-219). [[Image:Fatty Acyl Binding Crevice.jpg|thumb|200px|left|Fatty Acyl Binding Crevice (substrate binding loop in purple; substrates pictured inside the crevice)]] One side of this crevice is open and exposed to solvent, which allows the substrates to access the binding pocket of this enzyme. The size of the substrate binding loop is a primary determinant of the ability of InhA to select for fatty acyl chains longer than 16 carbons to successfully produce mycolic acid precursors. | Crystal structures of InhA reveal a <scene name='69/694241/Homotetramer_subunits_labeled/1'>homotetramer</scene> (each subunit featured with a different color) in aqueous solution with separate ligand binding sites in each subunit. Each <scene name='69/694241/Monomer_subunit_no_ligands/1'>monomer</scene> subunit is composed of 289 residues and features a typical [http://en.wikipedia.org/wiki/Rossmann_fold Rossmann fold] containing a single NADH binding site. The <scene name='69/694241/Secondary_structure_black/1'>secondary structure</scene> of InhA is made up of several alpha helices (pink), beta sheets (gold), and beta turns (white). This enzyme also features a fatty acyl binding crevice that accommodates the long-chain fatty acyl substrate needed to synthesize mycolic acid precursors. The <scene name='69/694241/Helix6_helix7_updated/1'>alpha-6 and alpha-7 helices</scene> of the InhA form one side of the fatty acyl binding crevice, referred to as the <scene name='69/694241/Monomer_subunit_196_219/1'> substrate binding loop</scene> (residues 196-219). [[Image:Fatty Acyl Binding Crevice.jpg|thumb|200px|left|Fatty Acyl Binding Crevice (substrate binding loop in purple; substrates pictured inside the crevice)]] One side of this crevice is open and exposed to solvent, which allows the substrates to access the binding pocket of this enzyme. The size of the substrate binding loop is a primary determinant of the ability of InhA to select for fatty acyl chains longer than 16 carbons to successfully produce mycolic acid precursors. | ||
| - | |||
| - | |||
| - | Talk generally about catalytic triad. More specific details in sections below. | ||
==='''Ligands'''=== | ==='''Ligands'''=== | ||
| Line 24: | Line 21: | ||
| - | + | == '''Fatty Acyl Binding Crevice''' == | |
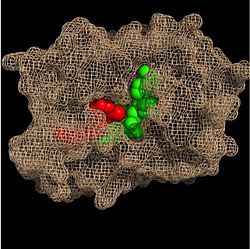
Within the fatty acyl binding crevice, the NADH substrate sits on the top shelf of the Rossmann fold, and the fatty acyl substrate sits on top of the NADH substrate. Due to its position within the crevice, the ''trans'' double bond of the fatty acyl substrate is found near the closed end of the crevice and is located directly adjacent to the nicotinamide ring of [http://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NADH]. [[Image:Binding Pocket - Mesh.jpg|thumb|250px|right|Substrate Binding Pocket (NADH in green; fatty acyl substrate in red)]] These two molecules interact with each other via hydrogen bonding. The first hydrogen bond occurs between the phosphate oxygen of NADH and the amide nitrogen of the N-acetylcysteamine portion of the fatty acyl substrate. The second hydrogen bond occurs between the 2'-hydroxyl of the nicotinamide ribose of NADH and the thioester carbonyl oxygen of the fatty acyl substrate. | Within the fatty acyl binding crevice, the NADH substrate sits on the top shelf of the Rossmann fold, and the fatty acyl substrate sits on top of the NADH substrate. Due to its position within the crevice, the ''trans'' double bond of the fatty acyl substrate is found near the closed end of the crevice and is located directly adjacent to the nicotinamide ring of [http://en.wikipedia.org/wiki/Nicotinamide_adenine_dinucleotide NADH]. [[Image:Binding Pocket - Mesh.jpg|thumb|250px|right|Substrate Binding Pocket (NADH in green; fatty acyl substrate in red)]] These two molecules interact with each other via hydrogen bonding. The first hydrogen bond occurs between the phosphate oxygen of NADH and the amide nitrogen of the N-acetylcysteamine portion of the fatty acyl substrate. The second hydrogen bond occurs between the 2'-hydroxyl of the nicotinamide ribose of NADH and the thioester carbonyl oxygen of the fatty acyl substrate. | ||
| Line 39: | Line 36: | ||
| - | + | == '''Catalytic Triad''' == | |
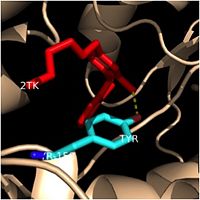
Structural studies have shown that InhA possesses the <scene name='69/694241/Catalytic_triad/1'> Phe-Tyr-Lys catalytic triad</scene>, composed of Phe-149, Tyr-158, and Lys-165. This catalytic triad of InhA is analogous to the classic Ser-Tyr-Lys catalytic triad of the SDR (Short-chain Dehydrogenase Reductase) family in terms of position and function within the enzyme. As discussed previously, the likely role of Tyr-158 is to position the fatty acyl substrate within the fatty acyl binding crevice. Second, the side chain of the Lys-165 residue in InhA functions similarly to the catalytic Lys residues in other SDR enzymes by interacting with the 3'-hydroxyl of the nicotinamide ring of NADH to hold this cofactor in place within the fatty acyl binding crevice. Finally, the exact role of the catalytic Phe-149 residue in InhA is unknown. It has been hypothesized to play a role in helping the fatty acyl substrate adopt its desired u-shape prior to binding. However, it is more widely believed that this catalytic residue merely distinguishes InhA as a dehydrogenase. | Structural studies have shown that InhA possesses the <scene name='69/694241/Catalytic_triad/1'> Phe-Tyr-Lys catalytic triad</scene>, composed of Phe-149, Tyr-158, and Lys-165. This catalytic triad of InhA is analogous to the classic Ser-Tyr-Lys catalytic triad of the SDR (Short-chain Dehydrogenase Reductase) family in terms of position and function within the enzyme. As discussed previously, the likely role of Tyr-158 is to position the fatty acyl substrate within the fatty acyl binding crevice. Second, the side chain of the Lys-165 residue in InhA functions similarly to the catalytic Lys residues in other SDR enzymes by interacting with the 3'-hydroxyl of the nicotinamide ring of NADH to hold this cofactor in place within the fatty acyl binding crevice. Finally, the exact role of the catalytic Phe-149 residue in InhA is unknown. It has been hypothesized to play a role in helping the fatty acyl substrate adopt its desired u-shape prior to binding. However, it is more widely believed that this catalytic residue merely distinguishes InhA as a dehydrogenase. | ||
Revision as of 02:44, 8 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Enoyl-ACP Reductase InhA from Mycobacterium tuberculosis
| |||||||||||