Sandbox Reserved 1074
From Proteopedia
(Difference between revisions)
| Line 36: | Line 36: | ||
== '''Catalytic Triad''' == | == '''Catalytic Triad''' == | ||
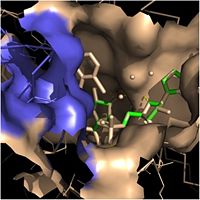
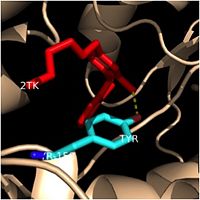
| - | Structural studies have shown that InhA possesses a <scene name='69/694241/Catalytic_triad/2'>Phe-Tyr-Lys catalytic triad</scene>, composed of Phe-149, Tyr-158, and Lys-165. This catalytic triad of InhA is analogous to the classic Ser-Tyr-Lys catalytic triad present in all members of the SDR (Short-chain Dehydrogenase Reductase) family (1). In the dehydrogenases, the first catalytic residue is usually Ser or Thr, while in the enoyl reuctases (InhA), the first catalytic residue is usually Phe or Tyr (2). According to crystallographic data, the likely role of Tyr-158 is to stabilize the enolate intermediate that forms during the hydride transfer reaction (1). Previous structural and kinetic studies have confirmed that the side chain of the Lys-165 residue in InhA functions similarly to the catalytic Lys residues in other SDR enzymes. The catalytic Lys-165 residue in InhA interacts with the 2' or 3'-hydroxyls of the nicotinamide ring of NADH to hold this cofactor in place within the fatty acyl binding crevice (1). Finally, the exact role of the catalytic Phe-149 residue in InhA is less well known. Recent Raman spectroscopy studies have supported that the catalytic Phe-149 residue plays an essential role in orienting the NADH cofactor correctly to lower the energy of the transition state and promoting the hydride transfer reaction. Altogether, the residues in the catalytic triad of InhA play critical roles in properly orienting NADH and the fatty acyl substrate to promote the hydride transfer reaction required to elongate the fatty acyl chains and produce mycolic acid precursors. | + | Structural studies have shown that InhA possesses a <scene name='69/694241/Catalytic_triad/2'>Phe-Tyr-Lys catalytic triad</scene>, composed of Phe-149, Tyr-158, and Lys-165. This catalytic triad of InhA is analogous to the classic Ser-Tyr-Lys catalytic triad present in all members of the SDR (Short-chain Dehydrogenase Reductase) family (1). In the dehydrogenases, the first catalytic residue is usually Ser or Thr, while in the enoyl reuctases (InhA), the first catalytic residue is usually Phe or Tyr (2). According to crystallographic data, the likely role of Tyr-158 is to stabilize the enolate intermediate that forms during the hydride transfer reaction (1). Previous structural and kinetic studies have confirmed that the side chain of the Lys-165 residue in InhA functions similarly to the catalytic Lys residues in other SDR enzymes. The catalytic Lys-165 residue in InhA interacts with the 2' or 3'-hydroxyls of the nicotinamide ring of NADH to hold this cofactor in place within the fatty acyl binding crevice (1). Finally, the exact role of the catalytic Phe-149 residue in InhA is less well known. Recent Raman spectroscopy studies have supported that the catalytic Phe-149 residue plays an essential role in orienting the NADH cofactor correctly to lower the energy of the transition state and promoting the hydride transfer reaction (2). Altogether, the residues in the catalytic triad of InhA play critical roles in properly orienting NADH and the fatty acyl substrate to promote the hydride transfer reaction required to elongate the fatty acyl chains and produce mycolic acid precursors. |
Revision as of 03:39, 8 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Enoyl-ACP Reductase InhA from Mycobacterium tuberculosis
| |||||||||||