We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1073
From Proteopedia
(Difference between revisions)
| Line 35: | Line 35: | ||
=== Other Inhibitors === | === Other Inhibitors === | ||
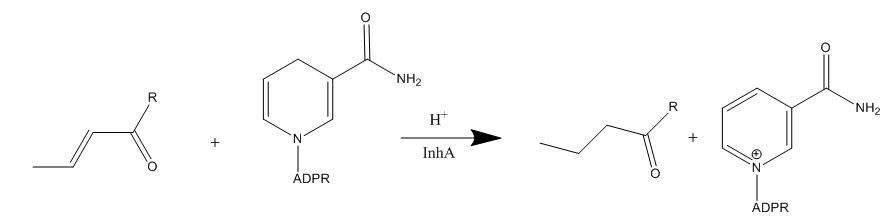
| - | Drug resistance of ''M. tuberculosis''has become a huge problem for the development of antibiotics. A drug screen of potential inhibitors of InhA (300 compounds), were composed of inhibitors of the '' | + | Drug resistance of ''M. tuberculosis''has become a huge problem for the development of antibiotics. A drug screen of potential inhibitors of InhA (300 compounds), were composed of inhibitors of the [http://en.wikipedia.org/wiki/Plasmodium_falciparum ''Plasmodium falciparum''] enoyl-reductase, against ''M. tuberculosis''. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 21:20, 8 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Enoyl-ACP Reductase InhA
| |||||||||||