Sandbox Reserved 1074
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
=== '''FAS-II System''' === | === '''FAS-II System''' === | ||
| - | [http://en.wikipedia.org/wiki/Mycolic_acid Mycolic acids] are very long-chain fatty acids (C<sub>60</sub> -C<sub>90</sub>) that are essential components of the mycobacterial cell wall. Mycolic acids are synthesized by at least two known elongation systems, type I and type II [http://en.wikipedia.org/wiki/Fatty_acid_synthase fatty acid synthases] (FAS-I and FAS-II) <ref name="FAS-II"> Bhatt, A. ''et al.'' (2007). The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. ''Journal of Molecular Microbiology, 64(6)'' 1442-1454. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/17555433 17555433] DOI: [http://www.ncbi.nlm.nih.gov/pubmed/17555433 10.1111/j.1365-2958.2007.05761.x]</ref>. The FAS-II system prefers C16 as a starting substrate and can extend up to C56. The FAS-II system utilizes the products from the FAS-I system as primers to extend the chain lengths further. The products of the FAS-II system are the precursors of mycolic acids. Elongation by the FAS-II system occurs by a [http://en.wikipedia.org/wiki/Condensation_reaction condensation reaction]of [http://en.wikipedia.org/wiki/Acetyl acetyl] and [http://en.wikipedia.org/wiki/Malonyl-CoA malonyl] substrates, which is achieved in three steps. Step 1 involves transfer of the acyl primer, step 2 involves [http://en.wikipedia.org/wiki/Decarboxylation decarboxylation] of the substrate to yield a [http://en.wikipedia.org/wiki/Carbanion carbanion], and step 3 involves nucleophilic attack of the carbanion to yield the elongated product <ref name="FAS-II" />. | + | [http://en.wikipedia.org/wiki/Mycolic_acid Mycolic acids] are very long-chain fatty acids (C<sub>60</sub> -C<sub>90</sub>) that are essential components of the mycobacterial cell wall. Mycolic acids are synthesized by at least two known elongation systems, type I and type II [http://en.wikipedia.org/wiki/Fatty_acid_synthase fatty acid synthases] (FAS-I and FAS-II) <ref name="FAS-II"> Bhatt, A. ''et al.'' (2007). The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. ''Journal of Molecular Microbiology, 64(6),'' 1442-1454. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/17555433 17555433] DOI: [http://www.ncbi.nlm.nih.gov/pubmed/17555433 10.1111/j.1365-2958.2007.05761.x]</ref>. The FAS-II system prefers C16 as a starting substrate and can extend up to C56 <ref name="FAS-II system"> Marrakchi, Hedia, ''et al.'' (2000). InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. ''Journal of Microbiology, 146,'' 289-296. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/10708367 10708367] </ref>. The FAS-II system utilizes the products from the FAS-I system as primers to extend the chain lengths further. The products of the FAS-II system are the precursors of mycolic acids. Elongation by the FAS-II system occurs by a [http://en.wikipedia.org/wiki/Condensation_reaction condensation reaction]of [http://en.wikipedia.org/wiki/Acetyl acetyl] and [http://en.wikipedia.org/wiki/Malonyl-CoA malonyl] substrates, which is achieved in three steps. Step 1 involves transfer of the acyl primer, step 2 involves [http://en.wikipedia.org/wiki/Decarboxylation decarboxylation] of the substrate to yield a [http://en.wikipedia.org/wiki/Carbanion carbanion], and step 3 involves nucleophilic attack of the carbanion to yield the elongated product <ref name="FAS-II" />. |
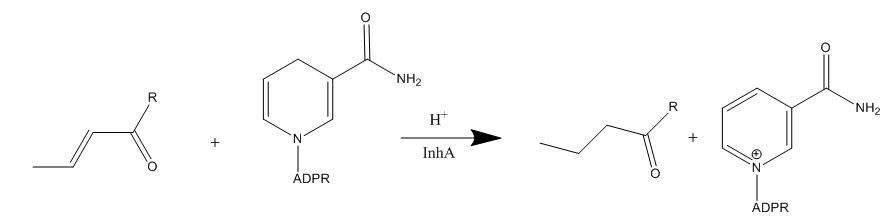
=== '''Mechanism of Action''' === | === '''Mechanism of Action''' === | ||
Revision as of 23:05, 8 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Enoyl-ACP Reductase InhA from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Bell, A.F. et al.(2007). Evidence from Raman Spectroscopy That InhA , the Mycobacterial Enoyl Reductase, Modulates the Conformation of the NADH Cofactor to Promote Catalysis. Journal of the American Chemical Society, 129, 6425-6431. DOI: 10.1021/ja068219m
- ↑ 2.0 2.1 Bhatt, A. et al. (2007). The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Journal of Molecular Microbiology, 64(6), 1442-1454. PMID: 17555433 DOI: 10.1111/j.1365-2958.2007.05761.x
- ↑ Marrakchi, Hedia, et al. (2000). InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Journal of Microbiology, 146, 289-296. PMID: 10708367
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Rozwarski, D.A. et al. (1999). Crystal Structure of the Mycobacterium tuberculosis Enoyl-ACP Reductase, InhA, in Complex with NAD+ and a C16 Fatty Acyl Substrate. Journal of Biological Chemistry, 274(22), 15582-15589. PMID: 10336454 DOI: 10.1074/jbc.274.22.15582