Sandbox Reserved 1074
From Proteopedia
(Difference between revisions)
| Line 57: | Line 57: | ||
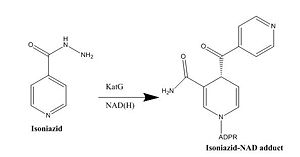
Drug resistance of ''M. tuberculosis'' has become a huge problem for the development of antibiotics. A drug screen of potential inhibitors of InhA (300 compounds) was composed of inhibitors of the [http://en.wikipedia.org/wiki/Plasmodium_falciparum ''Plasmodium falciparum''] enoyl-reductase. The enoyl reductases of both bacteria have limited similarities, however two compounds, CD39 and CD117 had activity against drug-susceptible ''M. tuberculosis''. More importantly, both compounds had activity against drug-resistant and multi-drug resistant TB. Treatment of the bacterium with the compounds resulted in the inhibition of mycolic acid and long-chain fatty acid biosynthesis, indicating that these compounds act against enzymes of both the FAS-I and FAS-II system. The benefit of having the compounds have multiple targets is the reduced development of drug resistance, which is the disadvantage of isoniazid. The essential chemical groups that lead to the antimycobacterial properties of the compounds include a [http://en.wikipedia.org/wiki/Thioacetic_acid thioacetate] group, and a [http://en.wikipedia.org/wiki/Butyl t-butyl] group <ref name="other inhibitors"> Vilchèze, C. ''et al.'' (2011). Novel Inhibitors of InhA Efficiently Kill Mycobacterium tuberculosis under Aerobic and Anaerobic Conditions. ''Antimicrobial Agents and Chemotherapy, 55(8),'' 3889-3898. DOI: [http://aac.asm.org/content/55/8/3889.full#T1 10.1128/AAC.00266-11]</ref>. | Drug resistance of ''M. tuberculosis'' has become a huge problem for the development of antibiotics. A drug screen of potential inhibitors of InhA (300 compounds) was composed of inhibitors of the [http://en.wikipedia.org/wiki/Plasmodium_falciparum ''Plasmodium falciparum''] enoyl-reductase. The enoyl reductases of both bacteria have limited similarities, however two compounds, CD39 and CD117 had activity against drug-susceptible ''M. tuberculosis''. More importantly, both compounds had activity against drug-resistant and multi-drug resistant TB. Treatment of the bacterium with the compounds resulted in the inhibition of mycolic acid and long-chain fatty acid biosynthesis, indicating that these compounds act against enzymes of both the FAS-I and FAS-II system. The benefit of having the compounds have multiple targets is the reduced development of drug resistance, which is the disadvantage of isoniazid. The essential chemical groups that lead to the antimycobacterial properties of the compounds include a [http://en.wikipedia.org/wiki/Thioacetic_acid thioacetate] group, and a [http://en.wikipedia.org/wiki/Butyl t-butyl] group <ref name="other inhibitors"> Vilchèze, C. ''et al.'' (2011). Novel Inhibitors of InhA Efficiently Kill Mycobacterium tuberculosis under Aerobic and Anaerobic Conditions. ''Antimicrobial Agents and Chemotherapy, 55(8),'' 3889-3898. DOI: [http://aac.asm.org/content/55/8/3889.full#T1 10.1128/AAC.00266-11]</ref>. | ||
| - | [[Image:CD39.JPG|thumb| | + | [[Image:CD39.JPG|thumb|450px|left|Figure 6. CD39 Structure<ref name="other inhibitors">]] [[Image:CD117.JPG|thumb|500 px|center|Figure 7. CD117 Structure<ref name="other inhibitors">]] |
Revision as of 23:54, 8 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
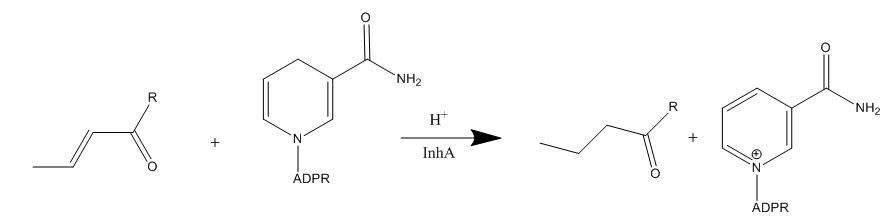
Enoyl-ACP Reductase InhA from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Bell, A.F. et al.(2007). Evidence from Raman Spectroscopy That InhA , the Mycobacterial Enoyl Reductase, Modulates the Conformation of the NADH Cofactor to Promote Catalysis. Journal of the American Chemical Society, 129, 6425-6431. DOI: 10.1021/ja068219m
- ↑ 2.0 2.1 Bhatt, A. et al. (2007). The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Journal of Molecular Microbiology, 64(6), 1442-1454. PMID: 17555433 DOI: 10.1111/j.1365-2958.2007.05761.x
- ↑ Marrakchi, Hedia, et al. (2000). InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Journal of Microbiology, 146, 289-296. PMID: 10708367
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Rozwarski, D.A. et al. (1999). Crystal Structure of the Mycobacterium tuberculosis Enoyl-ACP Reductase, InhA, in Complex with NAD+ and a C16 Fatty Acyl Substrate. Journal of Biological Chemistry, 274(22), 15582-15589. PMID: 10336454 DOI: 10.1074/jbc.274.22.15582
- ↑ 5.0 5.1 Vilchèze, C. et al. (2011). Novel Inhibitors of InhA Efficiently Kill Mycobacterium tuberculosis under Aerobic and Anaerobic Conditions. Antimicrobial Agents and Chemotherapy, 55(8), 3889-3898. DOI: 10.1128/AAC.00266-11