We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1066
From Proteopedia
(Difference between revisions)
| Line 13: | Line 13: | ||
= Mechanism = | = Mechanism = | ||
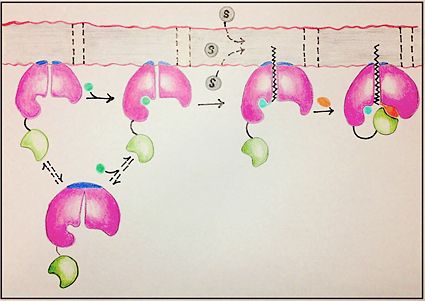
[[Image:FadD13 edited image.jpg|425 px|left|thumb|Figure 1: Mechanism for the activation of fatty acids (C24-C26) by FadD13. The N terminal domain (pink) is embedded in the membrane with the arginine rich lid-loop (dark blue), while the flexile linker (black) connects the C terminal domain (green) to the rest of the enzyme. Activation requires the binding of ATP (blue) which induces structural changes that promote the binding of the fatty acid chain. Formation of an acyl-adenylate intermediate induces a 140° rotation of the C terminal domain and the binding of CoA (orange). ]] | [[Image:FadD13 edited image.jpg|425 px|left|thumb|Figure 1: Mechanism for the activation of fatty acids (C24-C26) by FadD13. The N terminal domain (pink) is embedded in the membrane with the arginine rich lid-loop (dark blue), while the flexile linker (black) connects the C terminal domain (green) to the rest of the enzyme. Activation requires the binding of ATP (blue) which induces structural changes that promote the binding of the fatty acid chain. Formation of an acyl-adenylate intermediate induces a 140° rotation of the C terminal domain and the binding of CoA (orange). ]] | ||
| + | |||
| + | <scene name='69/694233/Lys_487/2'>Lys 487</scene> results in a 95% loss of function of FadD13. <ref name="Our Paper">PMID: PMC2793005</ref> | ||
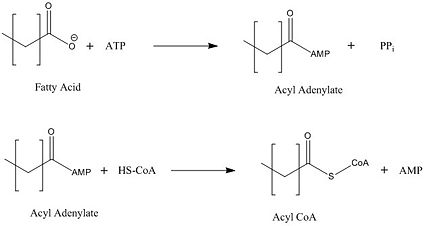
[[Image:acyl coa synthetase.jpg|425 px|left|thumb|Figure 2: Representation of the two-step reaction catalyzed by FadD13]] | [[Image:acyl coa synthetase.jpg|425 px|left|thumb|Figure 2: Representation of the two-step reaction catalyzed by FadD13]] | ||
| Line 40: | Line 42: | ||
== Active Site == | == Active Site == | ||
The active site on FadD13 is composed of two conserved regions, one of which serves as the binding site for ATP and the other for CoA. The adenine of ATP is bound to a group of <scene name='69/694232/Adenine_binding_group/2'>six amino acids (300-305)</scene> that is structurally identically to other acyl-CoA synthetases. <ref name="Our Paper"/> | The active site on FadD13 is composed of two conserved regions, one of which serves as the binding site for ATP and the other for CoA. The adenine of ATP is bound to a group of <scene name='69/694232/Adenine_binding_group/2'>six amino acids (300-305)</scene> that is structurally identically to other acyl-CoA synthetases. <ref name="Our Paper"/> | ||
| + | |||
| + | |||
= Disease = | = Disease = | ||
Revision as of 00:55, 9 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Mycobacterium tuberculosis very-long-chain fatty acyl-CoA synthetase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Andersson CS, Lundgren CA, Magnusdottir A, Ge C, Wieslander A, Molina DM, Hogbom M. The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: Structural Basis for Housing Lipid Substrates Longer than the Enzyme. Structure. 2012 May 2. PMID:22560731 doi:10.1016/j.str.2012.03.012
- ↑ Jatana N, Jangid S, Khare G, Tyagi AK, Latha N. Molecular modeling studies of Fatty acyl-CoA synthetase (FadD13) from Mycobacterium tuberculosis--a potential target for the development of antitubercular drugs. J Mol Model. 2011 Feb;17(2):301-13. doi: 10.1007/s00894-010-0727-3. Epub 2010 May, 8. PMID:20454815 doi:http://dx.doi.org/10.1007/s00894-010-0727-3
Similar Proteopedia Pages
Thioesterase Do we have anything like this?
External Resources
Tuberculosis Wikipedia page
Mycobacterium tuberculosis Wikipedia page
Coenzyme A Wikipedia page
Acyl CoA Wikipedia Page
Mycolic Acid Wikipedia page