We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1070
From Proteopedia
(Difference between revisions)
| Line 32: | Line 32: | ||
Support for a role in magnesium transport is supported by: 1) Mutants of MgtC are unable to survive in low-magnesium environment; 2) Expression of the gene encoding for MgtC is highly-induced in low magnesium environment; 3) Genes adjacent to the MgtC gene encode for known magnesium transporters. Very recent evidence against MgtC playing a role in magnesium transport showed that RT-PCR experiments gave consistent levels of MgtC expression despite changes in the concentration of extracellular magnesium. | Support for a role in magnesium transport is supported by: 1) Mutants of MgtC are unable to survive in low-magnesium environment; 2) Expression of the gene encoding for MgtC is highly-induced in low magnesium environment; 3) Genes adjacent to the MgtC gene encode for known magnesium transporters. Very recent evidence against MgtC playing a role in magnesium transport showed that RT-PCR experiments gave consistent levels of MgtC expression despite changes in the concentration of extracellular magnesium. | ||
| - | + | ||
===Potential for Binding Amino Acids=== | ===Potential for Binding Amino Acids=== | ||
The exploration of this role for MgtC was first considered because of the ACT domain-like structure of the C-terminal domain. | The exploration of this role for MgtC was first considered because of the ACT domain-like structure of the C-terminal domain. | ||
ACT domains commonly bind small amino acids within the cell as a form of regulation. Yang et al. showed that the structure | ACT domains commonly bind small amino acids within the cell as a form of regulation. Yang et al. showed that the structure | ||
of the C-terminal domain overlaps significantly with the structure of SerA, a known amino acid-binding ACT domain from ''E. coli'' | of the C-terminal domain overlaps significantly with the structure of SerA, a known amino acid-binding ACT domain from ''E. coli'' | ||
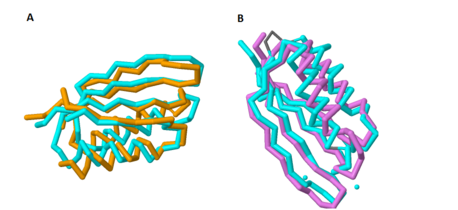
| - | + | Figure 1A shows the overlap of these two proteins; the blue protein represents MgtC and the orange protein represents SerA. | |
However, the glycine that is critical for the binding of amino acids in these ACT domains has been substituted in MgtC with a | However, the glycine that is critical for the binding of amino acids in these ACT domains has been substituted in MgtC with a | ||
tyrosine, likely abolishing any potential amino acid binding activity | tyrosine, likely abolishing any potential amino acid binding activity | ||
===Potential for Chelation=== | ===Potential for Chelation=== | ||
| - | As with the potential for binding amino acids, this role was also explored because of the structural similarity of the C-terminal domain with ACT domains, as ACT domains also serve as excellent chelators to sequester cations within the cell. Yang ''et al''. also compared the structure of the C-terminal domain of MgtC with an ACT domain of a known chelator, NikR. These structures overlapped quite well, indicating that MgtC may serve as a chelator. However, the two histidine residues and the cysteine residue present in NikR that serve as the chelating residues are modified to threonine, proline, and isoleucine respectively. These substitutions likely prevent any chelating activity by MgtC. | + | As with the potential for binding amino acids, this role was also explored because of the structural similarity of the C-terminal domain with ACT domains, as ACT domains also serve as excellent chelators to sequester cations within the cell. Yang ''et al''. also compared the structure of the C-terminal domain of MgtC with an ACT domain of a known chelator, NikR. These structures overlapped quite well, indicating that MgtC may serve as a chelator. Figure 1B highlights the significant overlap between these residues; the blue protein represents MgtC and the orange protein represents NikR. However, the two histidine residues and the cysteine residue present in NikR that serve as the chelating residues are modified to threonine, proline, and isoleucine respectively. These substitutions likely prevent any chelating activity by MgtC. |
| + | [[Image:Combined_overlaps.png |458 x 210 px|thumb|center|'''Figure 1. Overlap of the C-terminal Domain of MgtC with ACT domains of known function.''' 1A shows the significant overlap of the C-terminal of MgtC with SerA, an ACT domain that has been established to bind amino acids. 1B shows the overlap of the C-terminal domain of MgtC with NikR, a known chelating ACT domain.]] | ||
| + | |||
===Role in Dimerization=== | ===Role in Dimerization=== | ||
Revision as of 15:53, 9 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
MgtC: A Virulence Factor From Mycobacterium tuberculosis
| |||||||||||