We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1061
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_Butler_CH462_Sp2015_#}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
== Structure of ''Mycobacterium Tuberculosis'' NrdH == | == Structure of ''Mycobacterium Tuberculosis'' NrdH == | ||
| - | <StructureSection load=' | + | <StructureSection load='4hs1' size='300' side='right' caption='Micobacterium tuberculosis NrdH' scene=''> |
| - | + | == Overview == | |
| - | + | ''Mycobacterium tuberculosis'' NrdH is a small glutaredoxin-like protein involved in the electron transport chain that eventually leads to ribonucleotide reduction. | |
This page is for Bryant Dawson and Kate Burke hehehe | This page is for Bryant Dawson and Kate Burke hehehe | ||
| - | + | ||
| - | + | ||
| - | + | ||
== Background == | == Background == | ||
| Line 19: | Line 17: | ||
<ref>Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.</ref> | <ref>Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.</ref> | ||
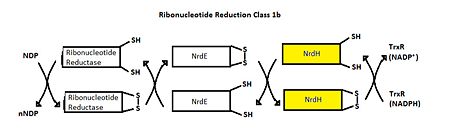
| - | [[Image:Ribonucleotide Reduction Class 1b.jpg|thumb|center|upright=2. | + | [[Image:Ribonucleotide Reduction Class 1b.jpg|thumb|center|upright=2.5|Ribonucleotide Reduction Class Ib general mechanism. The role of NrdH is highlighted.]] |
| Line 28: | Line 26: | ||
Many theirodoxin-like proteins have a similar active site region, denoted as the theirodoxin fold, which occurs directly before the disulfide bond. The residues in this region, denoted by letters CVQC, are the most highly conserved of all areas of the protein across multiple species. Exactly how this structure relates to function is somewhat debated. A Threonine-7 reside directly across the theirodoxin fold from the disulphide bond has been suggested to adopt two different conformations which differentially affect the redox abilities of the Protein. In the <scene name='69/694228/Nrdh_ligand_binding_site/8'>"A" Conformation</scene>, the alcohol of the threonine side chain points towards the disulfide bond, engaging an ionic interaction between the two that prevents the Therodoxin Reductase from binding. [[Image:Disulfide bond with ligand.png|thumb|ionic interaction between Thr-7 residue and disulfide bond which occurs across the theirodoxin fold]] Alternatively, in the <scene name='69/694228/Nrdh_ligand_binding_site/12'>"B" Conformation</scene>, the alcohol points in the opposite direction, allowing sufficient space for the ligand to bind and reduction to occur. | Many theirodoxin-like proteins have a similar active site region, denoted as the theirodoxin fold, which occurs directly before the disulfide bond. The residues in this region, denoted by letters CVQC, are the most highly conserved of all areas of the protein across multiple species. Exactly how this structure relates to function is somewhat debated. A Threonine-7 reside directly across the theirodoxin fold from the disulphide bond has been suggested to adopt two different conformations which differentially affect the redox abilities of the Protein. In the <scene name='69/694228/Nrdh_ligand_binding_site/8'>"A" Conformation</scene>, the alcohol of the threonine side chain points towards the disulfide bond, engaging an ionic interaction between the two that prevents the Therodoxin Reductase from binding. [[Image:Disulfide bond with ligand.png|thumb|ionic interaction between Thr-7 residue and disulfide bond which occurs across the theirodoxin fold]] Alternatively, in the <scene name='69/694228/Nrdh_ligand_binding_site/12'>"B" Conformation</scene>, the alcohol points in the opposite direction, allowing sufficient space for the ligand to bind and reduction to occur. | ||
| - | The active site of the protein is stabilized through a network of hydrogen bonds involving the two highly conserved residues, CVQC and WSGFRP. The crystal structure shows that interactions with one water molecule is necessary for the proper coordination between the conserved motifs to occur. These hydrogen bonds orient the important residues in the most optimal position to promote oxidation and reduction. | + | The active site of the protein is stabilized through a network of hydrogen bonds involving the two highly conserved residues, CVQC and WSGFRP. The crystal structure shows that interactions with one water molecule is necessary for the proper coordination between the conserved motifs to occur. These hydrogen bonds orient the important residues in the most optimal position to promote oxidation and reduction. |
== Function == | == Function == | ||
| Line 42: | Line 40: | ||
== Structural highlights == | == Structural highlights == | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | + | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.Jmol reference <ref>DOI 10.1002/ijch.201300024</ref> article describing Jmol <ref>PMID:21638687</ref> |
</StructureSection> | </StructureSection> | ||
Revision as of 00:13, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Structure of Mycobacterium Tuberculosis NrdH
| |||||||||||
References
- ↑ Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644