We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1061
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
''Mycobacterium tuberculosis'' [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Wikipedia]resides in the lungs of a host and upon becoming active, results in symptoms such as chest pains, weakness, and intense coughing. Left untreated and unmanaged, TB can lead to death (1.5 million in 2013). The disease has a high co-morbidity with HIV/AIDS due to its immunocompromising tendencies. Tuberculosis is one of the most heavily studied diseases today. With over 9 million infections worldwide per year, the necessity for antimicrobial agents to combat emerging multi-drug resistant strands is imperative. | ''Mycobacterium tuberculosis'' [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Wikipedia]resides in the lungs of a host and upon becoming active, results in symptoms such as chest pains, weakness, and intense coughing. Left untreated and unmanaged, TB can lead to death (1.5 million in 2013). The disease has a high co-morbidity with HIV/AIDS due to its immunocompromising tendencies. Tuberculosis is one of the most heavily studied diseases today. With over 9 million infections worldwide per year, the necessity for antimicrobial agents to combat emerging multi-drug resistant strands is imperative. | ||
| - | |||
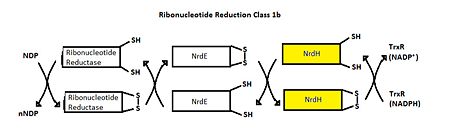
| - | MtNrdH has been identified as an electron carrier protein in ribonuleotide reduction. Ribonucleotide reduction uses an enzyme called ribonucleotide reductase (RNR) to make deoxyribonucleotides, which act as precursors to DNA synthesis. Three classes of RNRs have been identified; each class differs in cofactor requirement, structure, and oxygen dependence, but the general catalytic mechanism is conserved in all three classes. Mycobacterium tuberculosis uses class I ribonucleotide reductase. | ||
| - | |||
| - | Class I RNR is further subdivided into class Ia and Ib. Both Ia and Ib reduce ribonucleotide 5’ diphosphate to deoxyribonucleotide 5’ diphosphate (NDP to dNDP). After ribonucleotide reductase performs the first round of reduction, RNR must be reduced again to reset the cycle. In class Ib, RNR is reduced by either glutadoxin or thiordoxin, which are first reduced by glutadoxin reductase and thiordoxin reductase, respectively. In class Ib, RNR is reduced by NrdE, which is first reduced by NrdH. An important distinction between Ia and Ib is that Ia is present in eukaryotes, eubacteria, bacteriophages, and virus, but Ib is only present in eubacteria. | ||
| - | |||
| - | <ref>Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.</ref> | ||
| - | [[Image:Ribonucleotide Reduction Class 1b.jpg|thumb|center|upright=2.5|Ribonucleotide Reduction Class Ib general mechanism. The role of NrdH is highlighted.]] | ||
| Line 28: | Line 21: | ||
The active site of the protein is stabilized through a network of hydrogen bonds involving the two highly conserved residues, CVQC and WSGFRP. The crystal structure shows that interactions with one water molecule is necessary for the proper coordination between the conserved motifs to occur. These hydrogen bonds orient the important residues in the most optimal position to promote oxidation and reduction. | The active site of the protein is stabilized through a network of hydrogen bonds involving the two highly conserved residues, CVQC and WSGFRP. The crystal structure shows that interactions with one water molecule is necessary for the proper coordination between the conserved motifs to occur. These hydrogen bonds orient the important residues in the most optimal position to promote oxidation and reduction. | ||
| - | == Function == | ||
<scene name='69/694227/Arg_68_conformation_2/1'>TextToBeDisplayed</scene> | <scene name='69/694227/Arg_68_conformation_2/1'>TextToBeDisplayed</scene> | ||
| - | == | + | == Function == |
| + | MtNrdH has been identified as an electron carrier protein in ribonuleotide reduction. Ribonucleotide reduction uses an enzyme called ribonucleotide reductase (RNR) to make deoxyribonucleotides, which act as precursors to DNA synthesis. Three classes of RNRs have been identified; each class differs in cofactor requirement, structure, and oxygen dependence, but the general catalytic mechanism is conserved in all three classes. Mycobacterium tuberculosis uses class I ribonucleotide reductase. | ||
| + | |||
| + | Class I RNR is further subdivided into class Ia and Ib. Both Ia and Ib reduce ribonucleotide 5’ diphosphate to deoxyribonucleotide 5’ diphosphate (NDP to dNDP). After ribonucleotide reductase performs the first round of reduction, RNR must be reduced again to reset the cycle. In class Ib, RNR is reduced by either glutadoxin or thiordoxin, which are first reduced by glutadoxin reductase and thiordoxin reductase, respectively. In class Ib, RNR is reduced by NrdE, which is first reduced by NrdH. An important distinction between Ia and Ib is that Ia is present in eukaryotes, eubacteria, bacteriophages, and virus, but Ib is only present in eubacteria. | ||
| + | |||
| + | <ref>Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.</ref> | ||
| + | [[Image:Ribonucleotide Reduction Class 1b.jpg|thumb|center|upright=2.5|Ribonucleotide Reduction Class Ib general mechanism. The role of NrdH is highlighted.]] | ||
== Relevance == | == Relevance == | ||
Revision as of 00:16, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Structure of Mycobacterium Tuberculosis NrdH
| |||||||||||
References
- ↑ Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644