We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1058
From Proteopedia
(Difference between revisions)
| Line 57: | Line 57: | ||
Upregulation of the glyoxylate cycle has been seen for pathogens like ''M. tuberculosis'' that attack humans. <ref name="srivastava"> Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of ''Mycobacterium tuberculosis'' upregulated during residence in lungs of infected mice. ''ScienceDirect''. '''2007'''. doi:10.1016/j.tube.2007.10.002. </ref> Furthermore, isocitrate lyase has been found to be essential for tuberculosis survival within hosts. <ref name="muñoz-elías"> Muñoz-Elías, E.; McKinney, J.; ''M. tuberculosis'' isocitrate lyases 1 and 2 are jointly required for ''in vivo'' growth and virulence. ''Nat. Med.'' '''2005'''. ''11(6)'':638-644. doi:10.1038/nm1252. </ref> | Upregulation of the glyoxylate cycle has been seen for pathogens like ''M. tuberculosis'' that attack humans. <ref name="srivastava"> Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of ''Mycobacterium tuberculosis'' upregulated during residence in lungs of infected mice. ''ScienceDirect''. '''2007'''. doi:10.1016/j.tube.2007.10.002. </ref> Furthermore, isocitrate lyase has been found to be essential for tuberculosis survival within hosts. <ref name="muñoz-elías"> Muñoz-Elías, E.; McKinney, J.; ''M. tuberculosis'' isocitrate lyases 1 and 2 are jointly required for ''in vivo'' growth and virulence. ''Nat. Med.'' '''2005'''. ''11(6)'':638-644. doi:10.1038/nm1252. </ref> | ||
===Inhibitors=== | ===Inhibitors=== | ||
| - | Due to the increased usefulness of this enzyme in ''M. tuberculosis'' infections, specific inhibitors are being looked into as possible therapeutic targets for isocitrate lyase. Two such inhibitors that have already been identified are bromopyruvate and nitropropionate. Unfortunately, these molecules are non-specific and would also inhibit other enzymes essential for host function. <ref name="dunn"> Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. ''Microbiology''. '''2009'''. ''155'':3166-3175. doi:10.1099/mic.0.030858-0. </ref> More research is needed to identify inhibitors that selectively target enzymes in the glyoxylate cycle. | + | Due to the increased usefulness of this enzyme in propagating ''M. tuberculosis'' infections, specific inhibitors are being looked into as possible therapeutic targets for isocitrate lyase. Two such inhibitors that have already been identified are bromopyruvate and nitropropionate. Unfortunately, these molecules are non-specific and would also inhibit other enzymes essential for host function. <ref name="dunn"> Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. ''Microbiology''. '''2009'''. ''155'':3166-3175. doi:10.1099/mic.0.030858-0. </ref> More research is needed to identify inhibitors that selectively target enzymes in the glyoxylate cycle. |
Revision as of 01:08, 10 April 2015
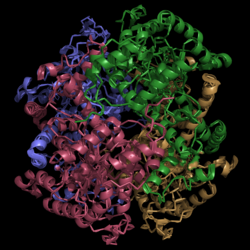
Isocitrate Lyase from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ Cozzone, A.; Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annual Review of Microbiology. 1998, 52:127-164. doi: 10.1146/annurev.micro.52.1.127.
- ↑ Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. ScienceDirect. 2007. doi:10.1016/j.tube.2007.10.002.
- ↑ Muñoz-Elías, E.; McKinney, J.; M. tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005. 11(6):638-644. doi:10.1038/nm1252.
- ↑ Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology. 2009. 155:3166-3175. doi:10.1099/mic.0.030858-0.