We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1058
From Proteopedia
(Difference between revisions)
| Line 6: | Line 6: | ||
===Structure=== | ===Structure=== | ||
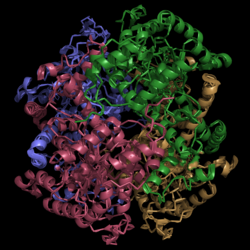
[[Image:Normal_Crystal_Structure.png|250 px|center|thumb|'''Figure 1. Crystal Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an alpha/beta barrel.]] | [[Image:Normal_Crystal_Structure.png|250 px|center|thumb|'''Figure 1. Crystal Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an alpha/beta barrel.]] | ||
| - | [http://www.rcsb.org/pdb/explore/explore.do?structureId=1f8i Isocitrate lyase] is a tetramer with 222 symmetry. Each subunit is composed of 14 alpha helices and 14 beta sheets which includes a total of 426 residues. These | + | [http://www.rcsb.org/pdb/explore/explore.do?structureId=1f8i Isocitrate lyase] is a tetramer with 222 symmetry. Each subunit is composed of 14 alpha helices and 14 beta sheets which includes a total of 426 residues. These α helices and β sheets form an unusual α/β barrel seen in Figure 1. The α/β barrel contains a topology of (βα)<sub>2</sub>α(βα)<sub>5</sub>β, differing from the canonical (βα)<sub>8</sub> pattern. Residues 184-200 and 235-254 connects the third and forth β-strands to their consecutive helices and form a small β-domain that consists of a short five-stranded βsheet(β6,β7,β9,β10,β11) that lies on top of the α/β barrel.*GREEN LINK MOTHERFUCKER* |
===Helix Swapping=== | ===Helix Swapping=== | ||
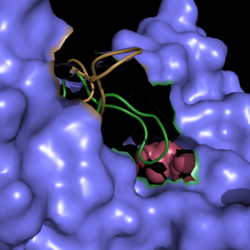
A unique structural feature of this enzyme is a phenomenon called "<scene name='69/694225/Helix_swapping/1'>helix swapping</scene>". | A unique structural feature of this enzyme is a phenomenon called "<scene name='69/694225/Helix_swapping/1'>helix swapping</scene>". | ||
| - | Helix swapping is observed between two monomers to form stable dimers. The | + | Helix swapping is observed between two monomers to form stable dimers. The 12th and 13th helices of each monomer exchange three dimensional placement with the respective helices of the opposite monomer. Due to the 222 symmetry observed, only two dimers form than combine to form the observed tetramer. As a result of this structure, 18% of the surface of each monomer is buried within the protein. |
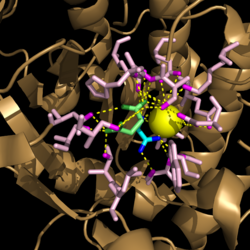
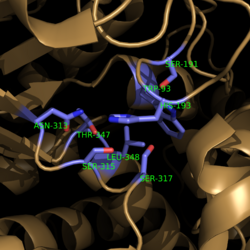
===Active Site Residues=== | ===Active Site Residues=== | ||
| Line 44: | Line 44: | ||
| - | ==3D Structures of Isocitrate Lyase== | + | ==Other 3D Structures of Isocitrate Lyase== |
*[http://www.rcsb.org/pdb/explore/explore.do?structureId=1F61 1F61] ''Mycobacterium tuberculosis'' | *[http://www.rcsb.org/pdb/explore/explore.do?structureId=1F61 1F61] ''Mycobacterium tuberculosis'' | ||
*[http://www.rcsb.org/pdb/explore/explore.do?structureId=1F8M 1F8M] ''Mycobacterium tuberculosis'' | *[http://www.rcsb.org/pdb/explore/explore.do?structureId=1F8M 1F8M] ''Mycobacterium tuberculosis'' | ||
Revision as of 03:41, 10 April 2015
Isocitrate Lyase from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ Cozzone, A.; Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annual Review of Microbiology. 1998, 52:127-164. doi: 10.1146/annurev.micro.52.1.127.
- ↑ Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. ScienceDirect. 2007. doi:10.1016/j.tube.2007.10.002.
- ↑ Muñoz-Elías, E.; McKinney, J.; M. tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005. 11(6):638-644. doi:10.1038/nm1252.
- ↑ Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology. 2009. 155:3166-3175. doi:10.1099/mic.0.030858-0.