We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1058
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
| - | =='''Isocitrate Lyase from ''Mycobacterium tuberculosis'''''== | + | β=='''Isocitrate Lyase from ''Mycobacterium tuberculosis'''''== |
<StructureSection load='1F8I' size='340' side='right' caption='Isocitrate Lyase' scene='> | <StructureSection load='1F8I' size='340' side='right' caption='Isocitrate Lyase' scene='> | ||
==Introduction== | ==Introduction== | ||
| Line 6: | Line 6: | ||
===Structure=== | ===Structure=== | ||
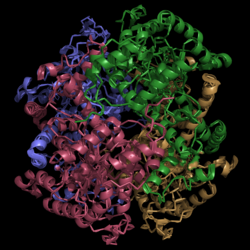
[[Image:Normal_Crystal_Structure.png|250 px|center|thumb|'''Figure 1. Crystal Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an alpha/beta barrel.]] | [[Image:Normal_Crystal_Structure.png|250 px|center|thumb|'''Figure 1. Crystal Structure of Isocitrate Lyase.''' Quaternary structure is comprised of four subunits forming an alpha/beta barrel.]] | ||
| - | [http://www.rcsb.org/pdb/explore/explore.do?structureId=1f8i Isocitrate lyase] is a tetramer with 222 symmetry. Each subunit is composed of 14 alpha helices and 14 beta sheets which includes a total of 426 residues. These α helices and β sheets form an unusual α/β barrel seen in Figure 1. The α/β barrel contains a topology of (βα)<sub>2</sub>α(βα)<sub>5</sub>β, differing from the canonical (βα)<sub>8</sub> pattern. Residues 184-200 and 235-254 connects the third and forth β-strands to their consecutive helices and form a small β-domain that consists of a short five-stranded βsheet (β6,β7,β9,β10,β11) that lies on top of the α/β barrel. | + | [http://www.rcsb.org/pdb/explore/explore.do?structureId=1f8i Isocitrate lyase] is a tetramer with 222 symmetry. Each subunit is composed of 14 alpha helices and 14 beta sheets which includes a total of 426 residues. These α helices and β sheets form an unusual α/β barrel seen in Figure 1. The α/β barrel contains a topology of (βα)<sub>2</sub>α(βα)<sub>5</sub>β, differing from the canonical (βα)<sub>8</sub> pattern. Residues 184-200 and 235-254 connects the third and forth β-strands to their consecutive helices and form a <scene name='69/694225/Small_beta_domain/1'>small β-domain</scene> that consists of a short five-stranded βsheet (β6,β7,β9,β10,β11) that lies on top of the α/β barrel. Isocitrate Lyase shows a resemblance to [http://www.rcsb.org/pdb/explore/explore.do?structureId=1S2V phosphoenolpyrvate mutase] |
===Helix Swapping=== | ===Helix Swapping=== | ||
A unique structural feature of this enzyme is a phenomenon called "<scene name='69/694225/Helix_swapping/1'>helix swapping</scene>". | A unique structural feature of this enzyme is a phenomenon called "<scene name='69/694225/Helix_swapping/1'>helix swapping</scene>". | ||
Revision as of 04:07, 10 April 2015
β==Isocitrate Lyase from Mycobacterium tuberculosis==
| |||||||||||
References
- ↑ Cozzone, A.; Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annual Review of Microbiology. 1998, 52:127-164. doi: 10.1146/annurev.micro.52.1.127.
- ↑ Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. ScienceDirect. 2007. doi:10.1016/j.tube.2007.10.002.
- ↑ Muñoz-Elías, E.; McKinney, J.; M. tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005. 11(6):638-644. doi:10.1038/nm1252.
- ↑ Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology. 2009. 155:3166-3175. doi:10.1099/mic.0.030858-0.