Sandbox Reserved 1058
From Proteopedia
(Difference between revisions)
| Line 34: | Line 34: | ||
==Mechanism of Action== | ==Mechanism of Action== | ||
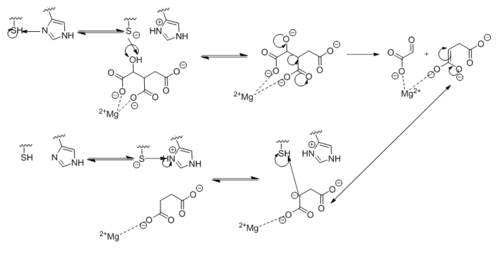
[[Image:Complete_Mechanism.PNG|500 px|right|thumb|'''Figure 6. Observed Mechanism for the Breakdown of Isocitrate by Isocitrate Lyase.''']] | [[Image:Complete_Mechanism.PNG|500 px|right|thumb|'''Figure 6. Observed Mechanism for the Breakdown of Isocitrate by Isocitrate Lyase.''']] | ||
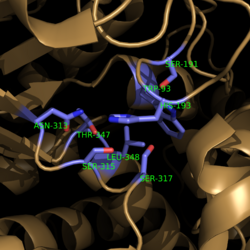
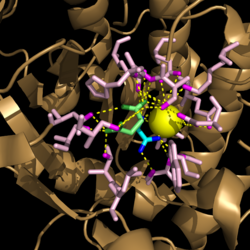
| + | His193 shifts the pKa of Cys191 and removes its proton. This allows Cys191 to extract a proton from the hydroxyl group of isocitrate. The resulting oxyanion forms a carbonyl and forces the lysis of a C-C bond. Glyoxylate and the enol form of succinate are formed and stabilized with a Mg<sup>2+</sup> ion. The succinate enolate resonates and extracts the proton back from Cys191 to form succinate (Figure 6). | ||
| - | His193 shifts the pKa of Cys191 and removes its proton. This allows Cys191 to extract a proton from the hydroxyl group of isocitrate. The resulting oxyanion forms a carbonyl and forces the lysis of a C-C bond. Glyoxylate and the enol form of succinate are formed and stabilized with a Mg<sup>2+</sup> ion. The succinate enolate resonates and extracts the proton back from Cys191 to form succinate (Figure 6). | ||
| Line 60: | Line 60: | ||
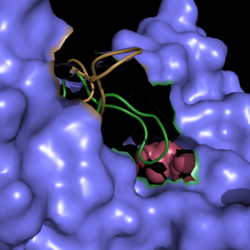
Isocitrate lyase plays a key role in survival of ''M. tuberculosis'' by sustaining intracellular infections in inflammatory respiratory macrophages. Used in the citric acid cycle, isocitrate lyase is the first enzyme catalyzing the carbon conserving glyoxylate pathway (Figure 7). This glyoxylate pathway has not been observed in mammals and thus presents a unique drug target to solely attack TB infections. | Isocitrate lyase plays a key role in survival of ''M. tuberculosis'' by sustaining intracellular infections in inflammatory respiratory macrophages. Used in the citric acid cycle, isocitrate lyase is the first enzyme catalyzing the carbon conserving glyoxylate pathway (Figure 7). This glyoxylate pathway has not been observed in mammals and thus presents a unique drug target to solely attack TB infections. | ||
Upregulation of the glyoxylate cycle has been seen for pathogens like ''M. tuberculosis'' that attack humans. <ref name="srivastava"> Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of ''Mycobacterium tuberculosis'' upregulated during residence in lungs of infected mice. ''ScienceDirect''. '''2007'''. doi:10.1016/j.tube.2007.10.002. </ref> Furthermore, isocitrate lyase has been found to be essential for tuberculosis survival within hosts. <ref name="muñoz-elías"> Muñoz-Elías, E.; McKinney, J.; ''M. tuberculosis'' isocitrate lyases 1 and 2 are jointly required for ''in vivo'' growth and virulence. ''Nat. Med.'' '''2005'''. ''11(6)'':638-644. doi:10.1038/nm1252. </ref> | Upregulation of the glyoxylate cycle has been seen for pathogens like ''M. tuberculosis'' that attack humans. <ref name="srivastava"> Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of ''Mycobacterium tuberculosis'' upregulated during residence in lungs of infected mice. ''ScienceDirect''. '''2007'''. doi:10.1016/j.tube.2007.10.002. </ref> Furthermore, isocitrate lyase has been found to be essential for tuberculosis survival within hosts. <ref name="muñoz-elías"> Muñoz-Elías, E.; McKinney, J.; ''M. tuberculosis'' isocitrate lyases 1 and 2 are jointly required for ''in vivo'' growth and virulence. ''Nat. Med.'' '''2005'''. ''11(6)'':638-644. doi:10.1038/nm1252. </ref> | ||
| + | |||
| + | |||
| + | |||
| + | |||
===Inhibitors=== | ===Inhibitors=== | ||
Due to the increased usefulness of this enzyme in propagating ''M. tuberculosis'' infections, specific inhibitors are being looked into as possible therapeutic targets for isocitrate lyase. Two such inhibitors that have already been identified are bromopyruvate and nitropropionate. Unfortunately, these molecules are non-specific and would also inhibit other enzymes essential for host function. <ref name="dunn"> Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. ''Microbiology''. '''2009'''. ''155'':3166-3175. doi:10.1099/mic.0.030858-0. </ref> More research is needed to identify inhibitors that selectively target enzymes in the glyoxylate cycle. | Due to the increased usefulness of this enzyme in propagating ''M. tuberculosis'' infections, specific inhibitors are being looked into as possible therapeutic targets for isocitrate lyase. Two such inhibitors that have already been identified are bromopyruvate and nitropropionate. Unfortunately, these molecules are non-specific and would also inhibit other enzymes essential for host function. <ref name="dunn"> Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. ''Microbiology''. '''2009'''. ''155'':3166-3175. doi:10.1099/mic.0.030858-0. </ref> More research is needed to identify inhibitors that selectively target enzymes in the glyoxylate cycle. | ||
Revision as of 05:22, 10 April 2015
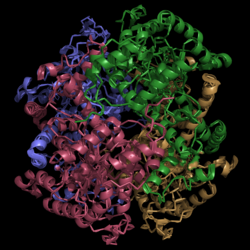
Isocitrate Lyase from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Sharma, V.; Sharma, S.; Hoener zu Bentrup, K.; McKinney, J.; Russell, D.; et. al; Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat. Struct. Biol.. 2000. 7(8):663-668.
- ↑ Gould, T.; van de Langemheen, H.; Muñoz-Elías, E.; McKinney, D.; Sacchettini, J.; Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium tuberculosis. Molecular Microbiology. 2006. 61(4):940-947. doi:10.1111/j.1365-2958.2006.05297.x.
- ↑ Cozzone, A.; Regulation of acetate metabolism by protein phosphorylation in enteric bacteria. Annual Review of Microbiology. 1998, 52:127-164. doi: 10.1146/annurev.micro.52.1.127.
- ↑ Srivastava, V.; Janin, A.; Srivastava, B.; Srivastava, R.; Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. ScienceDirect. 2007. doi:10.1016/j.tube.2007.10.002.
- ↑ Muñoz-Elías, E.; McKinney, J.; M. tuberculosis isocitrate lyases 1 and 2 are jointly required for in vivo growth and virulence. Nat. Med. 2005. 11(6):638-644. doi:10.1038/nm1252.
- ↑ Dunn, M.; Ramírez-Trujillo, J.; Hernández-Lucas, I.; Major roles of isocitrate lyase and malate synthase in bacterial and fungal pathogenesis. Microbiology. 2009. 155:3166-3175. doi:10.1099/mic.0.030858-0.