We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1061
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='4hs1' size='300' side='right' caption='Micobacterium tuberculosis NrdH' scene=''> | <StructureSection load='4hs1' size='300' side='right' caption='Micobacterium tuberculosis NrdH' scene=''> | ||
== Overview == | == Overview == | ||
| - | ''Mycobacterium tuberculosis'' NrdH is a small glutaredoxin-like protein involved in the electron transport chain in ribonucleotide reduction. | + | ''Mycobacterium tuberculosis'' NrdH is a small glutaredoxin-like protein involved in the electron transport chain in ribonucleotide reduction.<ref>Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.</ref> |
== Background == | == Background == | ||
| Line 20: | Line 20: | ||
[[Image:Weblogocvqc.png|thumb|center|upright=2.5|Weblogo diagram showing highly conserved CVQC region of NrdH.]] | [[Image:Weblogocvqc.png|thumb|center|upright=2.5|Weblogo diagram showing highly conserved CVQC region of NrdH.]] | ||
| - | Another highly conserved residue is the WSGFRP sequence. This nonpolar sequence is found on the surface of the molecule and is exposed to solvent. [[Image:Hydrophobic region pic.png|thumb| Hydrophobic region WSGFRP on the surface of MtNrdH (red) bound to ligand (green).]]For this reason, it has been hypothesized that this sequence plays a role in the binding of thioredoxin reductase. | + | Another highly conserved residue is the WSGFRP sequence. This nonpolar sequence is found on the surface of the molecule and is exposed to solvent. [[Image:Hydrophobic region pic.png|thumb| Hydrophobic region WSGFRP on the surface of MtNrdH (red) bound to ligand (green).]]<ref>DOI 10.1002/ijch.201300024</ref> <ref>PMID:21638687</ref> For this reason, it has been hypothesized that this sequence plays a role in the binding of thioredoxin reductase. <ref>Swastik, Phulera and Mande, Shekhar C. (2013) 4060.</ref> |
Arg-68 is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/3'>first conformation</scene>, Arg-68 is hydrogen bonded to His- 60 and Asp-59. When Arg-68 shifts to its <scene name='69/694227/Arg_68_conformation_2/2'>second conformation</scene> | Arg-68 is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/3'>first conformation</scene>, Arg-68 is hydrogen bonded to His- 60 and Asp-59. When Arg-68 shifts to its <scene name='69/694227/Arg_68_conformation_2/2'>second conformation</scene> | ||
| - | , it breaks it hydrogen bond with Asp-59. This reduction in hydrogen bonding gives the hydrophobic region more flexibility and is thought to occur when NrdH is in its inactive state. | + | , it breaks it hydrogen bond with Asp-59. <ref>Swastik, Phulera and Mande, Shekhar C. (2013) 4057.</ref> This reduction in hydrogen bonding gives the hydrophobic region more flexibility and is thought to occur when NrdH is in its inactive state. |
== Function == | == Function == | ||
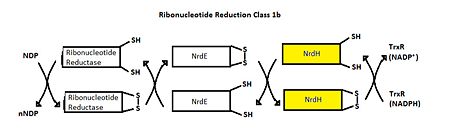
| - | MtNrdH has been identified as an electron carrier protein in ribonuleotide reduction. Ribonucleotide reduction uses an enzyme called ribonucleotide reductase (RNR) to make deoxyribonucleotides, which act as precursors to DNA synthesis. Three classes of RNRs have been identified; each class differs in cofactor requirement, structure, and oxygen dependence, but the general catalytic mechanism is conserved in all three classes. Mycobacterium tuberculosis uses class I ribonucleotide reductase. | + | MtNrdH has been identified as an electron carrier protein in ribonuleotide reduction. Ribonucleotide reduction uses an enzyme called ribonucleotide reductase (RNR) to make deoxyribonucleotides, which act as precursors to DNA synthesis. Three classes of RNRs have been identified; each class differs in cofactor requirement, structure, and oxygen dependence, but the general catalytic mechanism is conserved in all three classes.<ref>Swastik, Phulera and Mande, Shekhar C. (2013) 4056.</ref> Mycobacterium tuberculosis uses class I ribonucleotide reductase. |
| - | Class I RNR is further subdivided into class Ia and Ib. Both Ia and Ib reduce ribonucleotide 5’ diphosphate to deoxyribonucleotide 5’ diphosphate (NDP to dNDP). After ribonucleotide reductase performs the first round of reduction, RNR must be reduced again to reset the cycle. In class Ib, RNR is reduced by either glutadoxin or thiordoxin, which are first reduced by glutadoxin reductase and thiordoxin reductase, respectively. In class Ib, RNR is reduced by NrdE, which is first reduced by NrdH. An important distinction between Ia and Ib is that Ia is present in eukaryotes, eubacteria, bacteriophages, and virus, but Ib is only present in eubacteria. | + | Class I RNR is further subdivided into class Ia and Ib. Both Ia and Ib reduce ribonucleotide 5’ diphosphate to deoxyribonucleotide 5’ diphosphate (NDP to dNDP). After ribonucleotide reductase performs the first round of reduction, RNR must be reduced again to reset the cycle. In class Ib, RNR is reduced by either glutadoxin or thiordoxin, which are first reduced by glutadoxin reductase and thiordoxin reductase, respectively.<ref>Nelson, David L., and Michael M. Cox. Lehninger Principles of Biochemistry. 5th ed. New York: W.H. Freeman, 2008. 888-889.</ref> In class Ib, RNR is reduced by NrdE, which is first reduced by NrdH. An important distinction between Ia and Ib is that Ia is present in eukaryotes, eubacteria, bacteriophages, and virus, but Ib is only present in eubacteria. <ref>Swastik, Phulera and Mande, Shekhar C. (2013) 4056.</ref> |
<ref>Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.</ref> | <ref>Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.</ref> | ||
| Line 34: | Line 34: | ||
== Relevance == | == Relevance == | ||
| - | Like most NrdHs, MtNrdH is similar in sequence to glutaredoxins, but structurally similar to thioredoxins. MtNrdH also accepts electrons from thiordoxin reductase, a characteristic of thiordoxins, but not glutaredoxins. [[Image:Image-Super imposed molecules.png|thumb|left|Structural comparison of NrdHs with "thioredoxin folds": ''E. Coli'' NrdH (green), ''C. ammoniagenes'' NrdH (blue), ''M. tuberculosis'' NrdH (red)]] | + | Like most NrdHs, MtNrdH is similar in sequence to glutaredoxins, but structurally similar to thioredoxins. MtNrdH also accepts electrons from thiordoxin reductase, a characteristic of thiordoxins, but not glutaredoxins.<ref>Swastik, Phulera and Mande, Shekhar C. (2013) 4057.</ref> [[Image:Image-Super imposed molecules.png|thumb|left|Structural comparison of NrdHs with "thioredoxin folds": ''E. Coli'' NrdH (green), ''C. ammoniagenes'' NrdH (blue), ''M. tuberculosis'' NrdH (red)]]<ref>DOI 10.1002/ijch.201300024</ref> <ref>PMID:21638687</ref> |
Similar structures of NrdH have been isolated in other primitive species including ''E. coli'', ''S. pyogenes'', ''S. typhimurium'', ''D. deserti'', ''S. flexneri 2457T'', and ''S. dysenteriae''. In higher order multi-cellular organisms, however the NrdH protein is replaced by more complex glutaredoxins or theirodoxins. This observation leads some to speculate that NrdH is one of the very first ancestors in the ribonucleotide reduction pathway. If this is true, NrdH can be seen as a critical protein that allowed for the development of DNA-based life since deoxyribonucleotides could not have existed without the ribonucleotide reduction pathway. A better understanding of the evolutionary timeline of NrdH and similar proteins could shed greater light onto the RNA Wold Hypothesis, specifically describing the time frame of emergence of DNA based life. | Similar structures of NrdH have been isolated in other primitive species including ''E. coli'', ''S. pyogenes'', ''S. typhimurium'', ''D. deserti'', ''S. flexneri 2457T'', and ''S. dysenteriae''. In higher order multi-cellular organisms, however the NrdH protein is replaced by more complex glutaredoxins or theirodoxins. This observation leads some to speculate that NrdH is one of the very first ancestors in the ribonucleotide reduction pathway. If this is true, NrdH can be seen as a critical protein that allowed for the development of DNA-based life since deoxyribonucleotides could not have existed without the ribonucleotide reduction pathway. A better understanding of the evolutionary timeline of NrdH and similar proteins could shed greater light onto the RNA Wold Hypothesis, specifically describing the time frame of emergence of DNA based life. | ||
Revision as of 17:07, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Structure of Mycobacterium Tuberculosis NrdH
| |||||||||||
References
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4060.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4057.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4056.
- ↑ Nelson, David L., and Michael M. Cox. Lehninger Principles of Biochemistry. 5th ed. New York: W.H. Freeman, 2008. 888-889.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4056.
- ↑ Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4057.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644