From Proteopedia
(Difference between revisions)
proteopedia linkproteopedia link
|
|
| Line 6: |
Line 6: |
| | | | |
| | ==Introduction== | | ==Introduction== |
| - | ''Mycobacterim tuberculosis'' salicylate synthase (MtbI) is a highly promiscuous enzyme that has four distinct activities: isochorismate synthase (IS), isochorismate pyruvate lyase (IPL), salicylate synthase (SS) and chromate mutate (CM)(Figure 1). MtbI belongs to the MST enzyme family, which consists of structural homologues (Irp9, MenF, EntC, and MtbI) that isomerize chromate to isochorismate. These enzymes are in involved in menaquinone, siderophore, or tryptophan biosynthesis. The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating siderophore, mycobactin, in ''Mycobacterium tuberculosis''(Figure 3). | + | ''Mycobacterim tuberculosis'' salicylate synthase (MtbI) is a highly promiscuous enzyme that has four distinct activities: isochorismate synthase (IS), isochorismate pyruvate lyase (IPL), salicylate synthase (SS) and chromate mutate (CM)(Figure 1). MtbI belongs to the MST enzyme family, which consists of structural homologues (Irp9, MenF, EntC, and MtbI) that isomerize chromate to isochorismate. These enzymes are in involved in menaquinone, siderophore, or tryptophan biosynthesis <ref>PMID:23108268</ref>. The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating siderophore, mycobactin, in ''Mycobacterium tuberculosis''(Figure 3)<ref>PMID:22607697</ref>. |
| | | | |
| | [[Image:Pathways.png|500 px|center|thumb|Figure 1: This is the pathway of reactions catilized by wild-type MbtI. Ferrer ''et al.'', 2012]] | | [[Image:Pathways.png|500 px|center|thumb|Figure 1: This is the pathway of reactions catilized by wild-type MbtI. Ferrer ''et al.'', 2012]] |
Revision as of 17:55, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080.
|
To get started:
- Click the edit this page tab at the top. Save the page after each step, then edit it again.
- Click the 3D button (when editing, above the wikitext box) to insert Jmol.
- show the Scene authoring tools, create a molecular scene, and save it. Copy the green link into the page.
- Add a description of your scene. Use the buttons above the wikitext box for bold, italics, links, headlines, etc.
More help: Help:Editing
|
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| This is a default text for your page '. Click above on edit this page' to modify. Be careful with the < and > signs.
You may include any references to papers as in: the use of JSmol in Proteopedia [1] or to the article describing Jmol [2] to the rescue.
Introduction
Mycobacterim tuberculosis salicylate synthase (MtbI) is a highly promiscuous enzyme that has four distinct activities: isochorismate synthase (IS), isochorismate pyruvate lyase (IPL), salicylate synthase (SS) and chromate mutate (CM)(Figure 1). MtbI belongs to the MST enzyme family, which consists of structural homologues (Irp9, MenF, EntC, and MtbI) that isomerize chromate to isochorismate. These enzymes are in involved in menaquinone, siderophore, or tryptophan biosynthesis [3]. The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating siderophore, mycobactin, in Mycobacterium tuberculosis(Figure 3)[4].
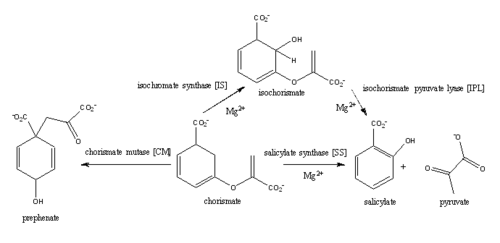 Figure 1: This is the pathway of reactions catilized by wild-type MbtI. Ferrer et al., 2012 Structure
Image:Active site cleft.png Figure 2: This shows a single sub unit of MbtI, with the active site cleft located at the lower left hand side of the image. The crystal asymmetric unit was found to contain , however crystal packing and size exclusion chromatography data suggest a monomeric enzyme. There are no significant structural changes between the four monomers excepts from the localized differences in the active site. The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes. The core of the protein is formed by 21 beta-strands folded into a twisted beta-sandwich. The protein's core is then surrounded by 10 alpha helices (Figure 2).
Disease
Mycobacterium tuberculosis is the causative agent of Tuberculosis (TB), an infectious disease that affects one-third of the worlds population. Iron is essential for mycobacterial growth and pathogenesis, M. tuberculosis obtains iron through mycobactin T, a type of siderophore. Mycobactin T is a hydrophilic molecule that has been proposed to be secreted actively into the aqueous growth medium for direct competition with iron-binding molecules of the environment. The core of the Mycobactin T siderophore is derived from salicylic acid which is synthesized from chorismate by salicylate synthase.
 Figure 3: Reaction catalyzed by MbtI in the mycobactin biosynthesis pathway. The salicylate synthase activity of MbtI produces salicylate from chorismate through an isochorismate intermediate. This reaction is Mg dependent. Chorismate is then used in the biosyntheses of Mycobactin T, the siderophore of M. tuberculosis. The siderophore sequesters iron, covering pathogenesis of M. tuberculosis.
Function
Relevance
Structural highlights
Mechanism
A magnesium ion in the active site orients the C1 carboxyl group of chorismate. A lysine residue then serves as a general base for the activation of a water molecule to attack at C2
The catalytic mechanism for conversion of isochorismate to salicylate by MbtI is a sigmatropic, pericyclic mechanism that is pH-dependent. Chromate mutase activity is only observed in the absence of magnesium ion in the active site while salicylate synthase activity is depended on magnesium ion. The active site of MbtI is altered by the removal of the magnesium cofactor causing chromate mutase activity. MbtI has differing binding modes for chromate that leads to different substrate conformations/transition states and resulting in different products.
Inhibition Studies
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
|
References
1. Chi G1, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. 2012. Implications of binding mode and active site flexibility for inhibitor potency against the salicylate synthase from Mycobacterium tuberculosis. Biochemistry 51(24):4868-79. doi: 10.1021/bi3002067
2. Ferrer S1, Martí S, Moliner V, Tuñón I, Bertrán J. 2012 Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 14(10):3482-9. doi: 10.1039/c2cp23149b.
3. Harrison AJ1, Yu M, Gårdenborg T, Middleditch M, Ramsay RJ, Baker EN, Lott JS. 2006. The structure of MbtI from Mycobacterium tuberculosis, the first enzyme in the biosynthesis of the siderophore mycobactin, reveals it to be a salicylate synthase. J Bacteriol. 188(17):6081-91.
4. Manos-Turvey A1, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. 2012. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem 10(46):9223-36. doi: 10.1039/c2ob26736e.
5. Zwahlen J1, Kolappan S, Zhou R, Kisker C, Tonge PJ. 2007. Structure and mechanism of MbtI, the salicylate synthase from Mycobacterium tuberculosis. Biochemistry. 46(4):954-64.


