Sandbox Reserved 1068
From Proteopedia
| Line 6: | Line 6: | ||
==Introduction== | ==Introduction== | ||
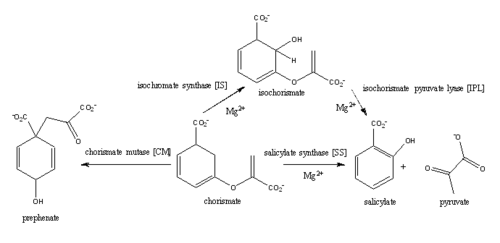
| - | ''Mycobacterim tuberculosis'' salicylate synthase (MtbI) is a highly promiscuous enzyme that has four distinct activities: isochorismate synthase (IS), isochorismate pyruvate lyase (IPL), salicylate synthase (SS) and chromate mutate (CM)(Figure 1). MtbI belongs to the MST enzyme family, which consists of structural homologues (<scene name='69/694235/Irp9/1'>Ipr9</scene>, <scene name='69/694235/Menf/1'>MenF</scene>, <scene name='69/694235/Entc/1'>EntC</scene>, and <scene name='69/694235/Mbti/1'>MbtI</scene>) that isomerize chromate to isochorismate. These enzymes are in involved in menaquinone, siderophore, or tryptophan biosynthesis <ref>PMID:23108268</ref>. The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating siderophore, mycobactin, in ''Mycobacterium tuberculosis''(Figure 3)<ref>PMID:22607697</ref>. This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''. Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action. | + | ''Mycobacterim tuberculosis'' salicylate synthase (MtbI) is a highly promiscuous enzyme that has four distinct activities: isochorismate synthase (IS), isochorismate pyruvate lyase (IPL), salicylate synthase (SS) and chromate mutate (CM)(Figure 1). MtbI belongs to the MST enzyme family, which consists of structural homologues (<scene name='69/694235/Irp9/1'>Ipr9</scene>, <scene name='69/694235/Menf/1'>MenF</scene>, <scene name='69/694235/Entc/1'>EntC</scene>, and <scene name='69/694235/Mbti/1'>MbtI</scene>) that isomerize chromate to isochorismate. These enzymes are in involved in menaquinone, siderophore, or tryptophan biosynthesis <ref>PMID:23108268</ref>. The salicylate synthase activity of MbtI catalyzes the first committed step in the synthesis of the iron chelating siderophore, mycobactin, in ''Mycobacterium tuberculosis''(Figure 3)<ref>PMID:22607697</ref>. This complex secondary metabolite is essential for both virulence and survival of ''M. tuberculosis''. Therefore, inhibitors of salicylate synthase may serve as potential TB therapies with a novel mode of action. The IS, IPL, and SS activity of MbtI are dependent on pH and the presence of the magnesium ion within the active site, while CM activity is only observed in absence of the magnesium cation. At pH values below 7.5 isochorismate is the main product while at pH values above 8 the main product is salicylate. |
[[Image:Pathways.png|500 px|center|thumb|Figure 1: This is the pathway of reactions catilized by wild-type MbtI. Ferrer ''et al.'', 2012]] | [[Image:Pathways.png|500 px|center|thumb|Figure 1: This is the pathway of reactions catilized by wild-type MbtI. Ferrer ''et al.'', 2012]] | ||
Revision as of 18:32, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
1. Chi G1, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. 2012. Implications of binding mode and active site flexibility for inhibitor potency against the salicylate synthase from Mycobacterium tuberculosis. Biochemistry 51(24):4868-79. doi: 10.1021/bi3002067
2. Ferrer S1, Martí S, Moliner V, Tuñón I, Bertrán J. 2012 Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 14(10):3482-9. doi: 10.1039/c2cp23149b.
3. Harrison AJ1, Yu M, Gårdenborg T, Middleditch M, Ramsay RJ, Baker EN, Lott JS. 2006. The structure of MbtI from Mycobacterium tuberculosis, the first enzyme in the biosynthesis of the siderophore mycobactin, reveals it to be a salicylate synthase. J Bacteriol. 188(17):6081-91.
4. Manos-Turvey A1, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. 2012. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem 10(46):9223-36. doi: 10.1039/c2ob26736e.
5. Zwahlen J1, Kolappan S, Zhou R, Kisker C, Tonge PJ. 2007. Structure and mechanism of MbtI, the salicylate synthase from Mycobacterium tuberculosis. Biochemistry. 46(4):954-64.