Sandbox Reserved 1053
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
== Methods of Inhibition == | == Methods of Inhibition == | ||
| + | |||
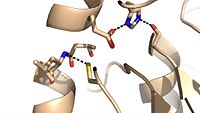
| + | [[Image:C209.jpeg|200 px|left|thumb|Cys209 stabilizing kinked formation of alpha-9 helix]] | ||
Due to the importance of Ag85C enzymatic activity in maintaining the integrity of the mycobacteria tuberculosis cell wall though mycolic acid modifications, the Ag85C enzyme represents a potentially effective avenue for inhibiting cell growth. The conformational sensitivity of the active site residues, H260, E228, and S124, relies entirely upon Van der Waals interaction between C209 and L232-T 231 (Figure #). The C209 facilitated interaction causes the <scene name='69/694220/Alpha_9_helix/2'>α9 helix</scene> to acquire a kinked conformation that promotes optimal interaction distances between catalytic residues. As a result, C209 has been a specific target residue for Ag85C inhibition. | Due to the importance of Ag85C enzymatic activity in maintaining the integrity of the mycobacteria tuberculosis cell wall though mycolic acid modifications, the Ag85C enzyme represents a potentially effective avenue for inhibiting cell growth. The conformational sensitivity of the active site residues, H260, E228, and S124, relies entirely upon Van der Waals interaction between C209 and L232-T 231 (Figure #). The C209 facilitated interaction causes the <scene name='69/694220/Alpha_9_helix/2'>α9 helix</scene> to acquire a kinked conformation that promotes optimal interaction distances between catalytic residues. As a result, C209 has been a specific target residue for Ag85C inhibition. | ||
Revision as of 18:54, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Background
The antigen 85 (ag85) complex in Mycobacterium tuberculosis is composed of three intracellular membrane proteins: ag85A, B, and C. The ag85 complex is a major component of the cell wall, with each protein catalyzing the transfer of important cell wall constituents into the membrane. [1] Ag85C is of particular interest due to its transfer of mycolic acids, which are major components in determining cell wall integrity. By targeting this mycoloyltransferase activity, inhibition of ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant mycotaberia tuberculosis. [2]
Structure
| |||||||||||