Sandbox Reserved 1053
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
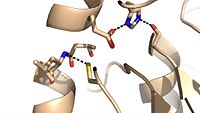
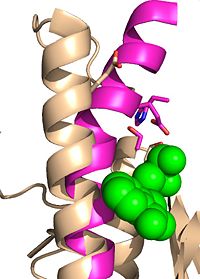
Ag85C can be inhibited by ebselen covalently bound to the sulfur of C209. Ebselen is a thiol-modifying agent that serves as an electrophile for the C209 that results in a sulfur-selenium bond. Co-crystallization of ebselen with Ag85C provides an explanation for the mechanism of ebselen-based inhibition. The addition of ebselen increases the distance between C209 and L232-T31, which effectively disrupts the interaction that holds the α9 helix in the active conformation. Furthermore, the bulk of ebselen creates steric hindrance with the α9 helix residues (Figure #). Relaxation of the α9 helix removes E228 and H260, which now interacts with S148, from the active site. The absence of these residues decreases the nucleophilicity of the S124 alcohol which decreases serine hydrolytic activity. | Ag85C can be inhibited by ebselen covalently bound to the sulfur of C209. Ebselen is a thiol-modifying agent that serves as an electrophile for the C209 that results in a sulfur-selenium bond. Co-crystallization of ebselen with Ag85C provides an explanation for the mechanism of ebselen-based inhibition. The addition of ebselen increases the distance between C209 and L232-T31, which effectively disrupts the interaction that holds the α9 helix in the active conformation. Furthermore, the bulk of ebselen creates steric hindrance with the α9 helix residues (Figure #). Relaxation of the α9 helix removes E228 and H260, which now interacts with S148, from the active site. The absence of these residues decreases the nucleophilicity of the S124 alcohol which decreases serine hydrolytic activity. | ||
| - | |||
| - | Thiol-modification reaction example | ||
Revision as of 18:55, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Background
The antigen 85 (ag85) complex in Mycobacterium tuberculosis is composed of three intracellular membrane proteins: ag85A, B, and C. The ag85 complex is a major component of the cell wall, with each protein catalyzing the transfer of important cell wall constituents into the membrane. [1] Ag85C is of particular interest due to its transfer of mycolic acids, which are major components in determining cell wall integrity. By targeting this mycoloyltransferase activity, inhibition of ag85C offers potential for cell wall disruption and subsequent antibiotic targeting for normally drug-resistant mycotaberia tuberculosis. [2]
Structure
| |||||||||||