We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1061
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
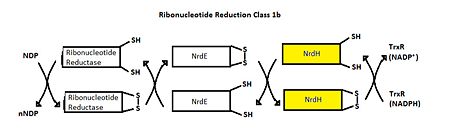
The structure of ''M. tuberculosis'' as determined by x-ray crystallography has 79 residues in a single polypeptide chain. <scene name='69/694228/Nrdh_structure/1'>NrdH Chrystal Structure</scene>. The active site (shown in green) is dominated by a disulfide bond between Cys-11 and Cys-14, which serves as the site of reduction by theirodoxin reductase. <ref>Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.</ref> | The structure of ''M. tuberculosis'' as determined by x-ray crystallography has 79 residues in a single polypeptide chain. <scene name='69/694228/Nrdh_structure/1'>NrdH Chrystal Structure</scene>. The active site (shown in green) is dominated by a disulfide bond between Cys-11 and Cys-14, which serves as the site of reduction by theirodoxin reductase. <ref>Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.</ref> | ||
| - | Many | + | Many thioredoxin-like proteins have a similar active site region, denoted as the thioredoxin fold, which occurs directly before the disulfide bond. The residues in this region, denoted by letters CVQC, are the most highly conserved of all areas of the protein across multiple species. Exactly how this structure relates to function is somewhat debated. A threonine-7 reside directly across the thioredoxin fold from the disulfide bond has been suggested to adopt two different conformations which differentially affect the redox abilities of the protein. In the <scene name='69/694228/Nrdh_ligand_binding_site/8'>"A" Conformation</scene>, the alcohol of the threonine side chain points towards the disulfide bond, engaging an ionic interaction between the two that prevents thioredoxin reductase from binding. Alternatively, in the <scene name='69/694228/Nrdh_ligand_binding_site/12'>"B" Conformation</scene>, the alcohol points in the opposite direction, allowing sufficient space for the ligand to bind and reduction to occur.<ref>Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.</ref> |
| - | The active site of the protein is stabilized through a <scene name='69/696879/Water_coordination/1'>hydrogen bond network</scene> involving the two highly conserved residues, CVQC and [http://www.proteopedia.org/wiki/index.php/Image:Weblogowsgfrp.png WSGFRP]. The crystal structure shows that interactions with one water molecule is necessary for the proper coordination between the conserved motifs to occur. These hydrogen bonds orient the important residues in the most optimal position to promote oxidation and reduction.<ref>Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.</ref> | + | The active site of the protein is stabilized through a <scene name='69/696879/Water_coordination/1'>hydrogen bond network</scene> involving the two highly conserved residues, CVQC (green) and [http://www.proteopedia.org/wiki/index.php/Image:Weblogowsgfrp.png WSGFRP] (yellow). The crystal structure shows that interactions with one water molecule is necessary for the proper coordination between the conserved motifs to occur. These hydrogen bonds orient the important residues in the most optimal position to promote oxidation and reduction.<ref>Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.</ref> |
[[Image:Weblogocvqc.png|thumb|center|upright=2.5|Weblogo diagram showing highly conserved CVQC region of NrdH.]] | [[Image:Weblogocvqc.png|thumb|center|upright=2.5|Weblogo diagram showing highly conserved CVQC region of NrdH.]] | ||
| Line 23: | Line 23: | ||
Another highly conserved residue is the WSGFRP sequence. This nonpolar sequence is found on the surface of the molecule and is exposed to solvent. [[Image:Hydrophobic region pic.png|thumb| Hydrophobic region WSGFRP on the surface of MtNrdH (red) bound to ligand (green).]]<ref>DOI 10.1002/ijch.201300024</ref> <ref>PMID:21638687</ref> For this reason, it has been hypothesized that this sequence plays a role in the binding of thioredoxin reductase. <ref>Swastik, Phulera and Mande, Shekhar C. (2013) 4060.</ref> | Another highly conserved residue is the WSGFRP sequence. This nonpolar sequence is found on the surface of the molecule and is exposed to solvent. [[Image:Hydrophobic region pic.png|thumb| Hydrophobic region WSGFRP on the surface of MtNrdH (red) bound to ligand (green).]]<ref>DOI 10.1002/ijch.201300024</ref> <ref>PMID:21638687</ref> For this reason, it has been hypothesized that this sequence plays a role in the binding of thioredoxin reductase. <ref>Swastik, Phulera and Mande, Shekhar C. (2013) 4060.</ref> | ||
Arg-68 is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/3'>first conformation</scene>, Arg-68 is hydrogen bonded to His- 60 and Asp-59. When Arg-68 shifts to its <scene name='69/694227/Arg_68_conformation_2/2'>second conformation</scene> | Arg-68 is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/3'>first conformation</scene>, Arg-68 is hydrogen bonded to His- 60 and Asp-59. When Arg-68 shifts to its <scene name='69/694227/Arg_68_conformation_2/2'>second conformation</scene> | ||
| - | , it breaks | + | , it breaks its hydrogen bond with Asp-59. <ref>Swastik, Phulera and Mande, Shekhar C. (2013) 4057.</ref> This reduction in hydrogen bonding gives the hydrophobic region more flexibility and is thought to occur when NrdH is in its inactive state. |
== Function == | == Function == | ||
Revision as of 18:59, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Structure of Mycobacterium Tuberculosis NrdH
| |||||||||||
References
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ "Tuberculosis." Media Centre. World Health Organization, Web. 16 Mar. 2015. Media Centre. <http://www.who.int/mediacentre/factsheets/fs104/en/>.
- ↑ "Tuberculosis." Media Centre. World Health Organization, Web. 16 Mar. 2015. Media Centre. <http://www.who.int/mediacentre/factsheets/fs104/en/>.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4060.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4057.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4056.
- ↑ Nelson, David L., and Michael M. Cox. Lehninger Principles of Biochemistry. 5th ed. New York: W.H. Freeman, 2008. 888-889.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4056.
- ↑ Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) 4057.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644