Sandbox Reserved 1070
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='2lqj' size='340' side='right' caption='C-terminal Domain of Mg2+ transport P-type ATPase C (PDB: [http://www.rcsb.org/pdb/explore.do?structureId=2lqj 2LQJ])' scene='69/698113/Rainbow-colored_spectrum/2'> | <StructureSection load='2lqj' size='340' side='right' caption='C-terminal Domain of Mg2+ transport P-type ATPase C (PDB: [http://www.rcsb.org/pdb/explore.do?structureId=2lqj 2LQJ])' scene='69/698113/Rainbow-colored_spectrum/2'> | ||
==Introduction== | ==Introduction== | ||
| - | Tuberculosis, caused by ''[[Mycobacterium tuberculosis]]'', is a respiratory infection still prevalent throughout the world. During the last decade, the emergence of multi-drug resistant strains of ''M. tuberculosis'' has given rise to the need for the development of new antibiotics in order to combat the infection<ref>Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269.</ref>. In order to develop an efficacious antibiotic, the drug must be able to target a unique aspect of the bacteria, such as a protein, that is critical for its full virulence and survival. MgtC, an integral protein embedded in the extracellular membrane of ''M. tuberculosis'', has recently been hypothesized as a novel drug target to resolve tuberculosis infections. The targeting of MgtC was a result of observing that upon deletion of the protein from ''M. tuberculosis'', the bacteria are no longer able to survive due to inhibition of intramacrophage growth. <ref name="mgtc">Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263.</ref> | + | [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis], caused by ''[[Mycobacterium tuberculosis]]'', is a respiratory infection still prevalent throughout the world. During the last decade, the emergence of [http://en.wikipedia.org/wiki/Multiple_drug_resistance multi-drug resistant] strains of ''M. tuberculosis'' has given rise to the need for the development of new antibiotics in order to combat the infection<ref>Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269.</ref>. In order to develop an efficacious antibiotic, the drug must be able to target a unique aspect of the bacteria, such as a protein, that is critical for its full virulence and survival. MgtC, an integral protein embedded in the extracellular membrane of ''M. tuberculosis'', has recently been hypothesized as a novel drug target to resolve tuberculosis infections. The targeting of MgtC was a result of observing that upon deletion of the protein from ''M. tuberculosis'', the bacteria are no longer able to survive due to inhibition of intramacrophage growth. <ref name="mgtc">Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263.</ref> |
== Structure == | == Structure == | ||
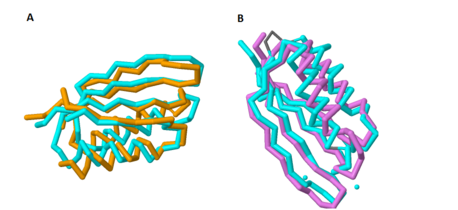
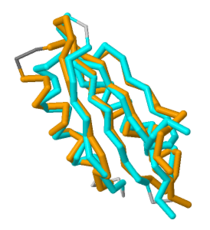
Based on its tertiary structure, this protein has been placed into a larger group of proteins known as the MgtC superfamily. The overall structure of MgtC is constituted by two domains: an N-terminal domain and a C-terminal domain. Each of these domains have striking similarities and differences with other MgtC-like proteins.<ref name="mgtc"/> | Based on its tertiary structure, this protein has been placed into a larger group of proteins known as the MgtC superfamily. The overall structure of MgtC is constituted by two domains: an N-terminal domain and a C-terminal domain. Each of these domains have striking similarities and differences with other MgtC-like proteins.<ref name="mgtc"/> | ||
Revision as of 23:21, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
MgtC: A Virulence Factor From Mycobacterium tuberculosis
| |||||||||||
References
- ↑ Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263.
- ↑ 3.0 3.1 Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study.