We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1070
From Proteopedia
(Difference between revisions)
| Line 72: | Line 72: | ||
The exploration of this role for MgtC was first considered because of the ACT domain-like structure of the C-terminal domain. | The exploration of this role for MgtC was first considered because of the ACT domain-like structure of the C-terminal domain. | ||
ACT domains commonly bind small amino acids within the cell as a form of regulation. Yang et al. showed that the structure | ACT domains commonly bind small amino acids within the cell as a form of regulation. Yang et al. showed that the structure | ||
| - | of the C-terminal domain overlaps significantly with the structure of SerA (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=1psd 1PSD]), a known amino acid-binding ACT domain from ''E. coli'' | + | of the C-terminal domain overlaps significantly with the structure of [http://proteopedia.org/wiki/index.php/1psd SerA] (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=1psd 1PSD]), a known amino acid-binding ACT domain from ''[http://www.cdc.gov/ecoli/ E. coli]'', |
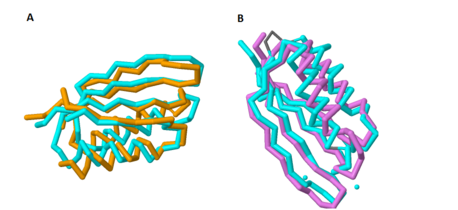
Figure 1A shows the overlap of these two proteins; the blue protein represents MgtC and the orange protein represents SerA. | Figure 1A shows the overlap of these two proteins; the blue protein represents MgtC and the orange protein represents SerA. | ||
However, the glycine that is critical for the binding of amino acids in these ACT domains has been substituted in MgtC with a <scene name='69/698113/Sub_residues_of_sera/1'>tyrosine</scene>, likely abolishing any potential amino acid binding activity <ref name="mgtc"/> | However, the glycine that is critical for the binding of amino acids in these ACT domains has been substituted in MgtC with a <scene name='69/698113/Sub_residues_of_sera/1'>tyrosine</scene>, likely abolishing any potential amino acid binding activity <ref name="mgtc"/> | ||
| Line 78: | Line 78: | ||
===Potential for Chelation=== | ===Potential for Chelation=== | ||
| - | As with the potential for binding amino acids, this role was also explored because of the structural similarity of the C-terminal domain with ACT domains, as ACT domains also serve as excellent chelators to sequester cations within the cell. Yang ''et al''. also compared the structure of the C-terminal domain of MgtC with an ACT domain of a known chelator, NikR (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=3LGH 3LGH]). These structures overlapped quite well, indicating that MgtC may serve as a chelator. Figure 1B highlights the significant overlap between these residues; the blue protein represents MgtC and the orange protein represents NikR. However, the two histidine residues and the cysteine residue present in NikR that serve as the chelating residues are modified to <scene name='69/698113/Sub_residues_of_chelat/2'>threonine, proline, and isoleucine</scene> respectively. These substitutions likely prevent any chelating activity by MgtC. <ref name="mgtc"/> | + | As with the potential for binding amino acids, this role was also explored because of the structural similarity of the C-terminal domain with ACT domains, as ACT domains also serve as excellent [http://en.wikipedia.org/wiki/Chelation chelators] to sequester cations within the cell. Yang ''et al''. also compared the structure of the C-terminal domain of MgtC with an ACT domain of a known chelator, [http://proteopedia.org/wiki/index.php/3lgh NikR] (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=3LGH 3LGH]). These structures overlapped quite well, indicating that MgtC may serve as a chelator. Figure 1B highlights the significant overlap between these residues; the blue protein represents MgtC and the orange protein represents NikR. However, the two histidine residues and the cysteine residue present in NikR that serve as the chelating residues are modified to <scene name='69/698113/Sub_residues_of_chelat/2'>threonine, proline, and isoleucine</scene> respectively. These substitutions likely prevent any chelating activity by MgtC. <ref name="mgtc"/> |
[[Image:Combined_overlaps.png |458 x 210 px|thumb|center|'''Figure 1. Overlap of the C-terminal Domain of MgtC with ACT domains of known function.''' 1A shows the significant overlap of the C-terminal of MgtC with SerA, an ACT domain that has been established to bind amino acids. 1B shows the overlap of the C-terminal domain of MgtC with NikR, a known chelating ACT domain.]] | [[Image:Combined_overlaps.png |458 x 210 px|thumb|center|'''Figure 1. Overlap of the C-terminal Domain of MgtC with ACT domains of known function.''' 1A shows the significant overlap of the C-terminal of MgtC with SerA, an ACT domain that has been established to bind amino acids. 1B shows the overlap of the C-terminal domain of MgtC with NikR, a known chelating ACT domain.]] | ||
===Role in Dimerization=== | ===Role in Dimerization=== | ||
| - | The potential for dimerization was another aspect of MgtC which was studied to see if this protein forms complexes with proteins of known function. A Bacterial Two-Hybrid (BACTH) assay was performed to study the potential for the entire protein to dimerize with itself and the potential for individual domains to dimerize. The results of this assay showed that the entire MgtC protein likely dimerizes, but the individual domains do not. This dimerization could serve as a critical component to the biochemical function of MgtC, although the exact implications have not yet been discerned <ref name="mgtc"/>. Frantz ''et al'' proposed a role for MgtC to form dimers with MgtR (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=2MC7 2MC7]), a protein that serves to promote the degradation of MgtC. This has huge implications in the overall clinical relevance of how MgtC could be targeted to develop new-generation antibiotics. <ref name="mgtr">Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study.</ref> | + | The potential for [http://en.wikipedia.org/wiki/Protein_dimer dimerization] was another aspect of MgtC which was studied to see if this protein forms complexes with proteins of known function. A [http://subtiwiki.uni-goettingen.de/wiki/index.php/BACTH Bacterial Two-Hybrid (BACTH)] assay was performed to study the potential for the entire protein to dimerize with itself and the potential for individual domains to dimerize. The results of this assay showed that the entire MgtC protein likely dimerizes, but the individual domains do not. This dimerization could serve as a critical component to the biochemical function of MgtC, although the exact implications have not yet been discerned <ref name="mgtc"/>. Frantz ''et al'' proposed a role for MgtC to form dimers with [http://proteopedia.org/wiki/index.php/2mc7 MgtR] (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=2MC7 2MC7]), a protein that serves to promote the degradation of MgtC. This has huge implications in the overall clinical relevance of how MgtC could be targeted to develop new-generation antibiotics. <ref name="mgtr">Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study.</ref> |
==Clinical Relevance == | ==Clinical Relevance == | ||
| - | The development of an antibiotic which targets and inhibits MgtC could come from exploitation and enhancement of the process which promotes its degradation within ''Mycobacterium tuberculosis.'' MgtR, a hydrophobic peptide, promotes the degradation of MgtC upon high expression with the bacteria. As previously stated, inadequate levels of MgtC within ''M. tuberculosis'' results in an inability to growth and survive. <ref name="mgtr"/> It is quite reasonable that analogues of MgtR could be developed, injected (subcutaneously) into infected patients, and resolve the tuberculosis infection by promoting degradation of MgtC and impairing growth of ''M. tuberculosis.'' | + | The development of an antibiotic which targets and inhibits MgtC could come from exploitation and enhancement of the process which promotes its degradation within ''Mycobacterium tuberculosis.'' MgtR, a hydrophobic peptide, promotes the degradation of MgtC upon high expression with the bacteria. As previously stated, inadequate levels of MgtC within ''M. tuberculosis'' results in an inability to growth and survive. <ref name="mgtr"/> It is quite reasonable that analogues of MgtR could be developed, injected ([http://en.wikipedia.org/wiki/Subcutaneous_injection subcutaneously]) into infected patients, and resolve the tuberculosis infection by promoting degradation of MgtC and impairing growth of ''M. tuberculosis.'' |
==Future Work== | ==Future Work== | ||
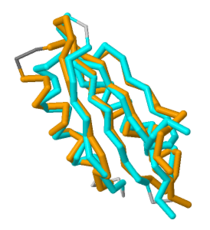
[[Image:Aligned 3.png|210 px|thumb|right|'''Figure 2. Overlap of MgtC C-terminal domain with the ACT domain of a GTP pyrophosphokinase.''' This figure demonstrates the significant overlap between the C-terminal domain of MgtC and the ACT domain of a GTP pyrophosphokinase.]] | [[Image:Aligned 3.png|210 px|thumb|right|'''Figure 2. Overlap of MgtC C-terminal domain with the ACT domain of a GTP pyrophosphokinase.''' This figure demonstrates the significant overlap between the C-terminal domain of MgtC and the ACT domain of a GTP pyrophosphokinase.]] | ||
| - | Since so little is known about MgtC, future work should involve both crystallizing the entire MgtC protein and characterizing its biochemical function. Because the sequence of amino acids in a protein dictates structure, and structure typically determines the protein's function, further sequencing and structural analysis should be performed with MgtC to discern its function. Shown in '''Figure 2''' is an overlap of MgtC with the ACT domain of a GTP pyrophosphokinase. This overlap shows even more extensive similarity than the aforementioned SerA and NikR ACT domains. Structural similarity analysis could aid in resolving the biochemical function of MgtC. | + | Since so little is known about MgtC, future work should involve both crystallizing the entire MgtC protein and characterizing its biochemical function. Because the sequence of amino acids in a protein dictates structure, and structure typically determines the protein's function, further sequencing and structural analysis should be performed with MgtC to discern its function. Shown in '''Figure 2''' is an overlap of MgtC with the ACT domain of a [http://en.wikipedia.org/wiki/GTP_diphosphokinase GTP pyrophosphokinase] (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=2kO1 2KO1]). This overlap shows even more extensive similarity than the aforementioned SerA and NikR ACT domains. Structural similarity analysis could aid in resolving the biochemical function of MgtC. |
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 23:59, 10 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
MgtC: A Virulence Factor From Mycobacterium tuberculosis
| |||||||||||
References
- ↑ Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263.
- ↑ 3.0 3.1 Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study.