We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1070
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='2lqj' size='340' side='right' caption='C-terminal Domain of Mg2+ transport P-type ATPase C (PDB: [http://www.rcsb.org/pdb/explore.do?structureId=2lqj 2LQJ])' scene='69/698113/Rainbow-colored_spectrum/2'> | <StructureSection load='2lqj' size='340' side='right' caption='C-terminal Domain of Mg2+ transport P-type ATPase C (PDB: [http://www.rcsb.org/pdb/explore.do?structureId=2lqj 2LQJ])' scene='69/698113/Rainbow-colored_spectrum/2'> | ||
==Introduction== | ==Introduction== | ||
| - | [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis], caused by ''[[Mycobacterium tuberculosis]]'', is a respiratory infection still prevalent throughout the world. During the last decade, the emergence of [http://en.wikipedia.org/wiki/Multiple_drug_resistance multi-drug resistant] strains of ''M. tuberculosis'' has given rise to the need for the development of new antibiotics in order to combat the infection<ref>Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269.</ref>. In order to develop an efficacious antibiotic, the drug must be able to target a unique aspect of the bacteria, such as a protein, that is critical for its full virulence and survival. MgtC, an [http://en.wikipedia.org/wiki/Integral_membrane_protein integral protein] embedded in the extracellular membrane of ''M. tuberculosis'', has recently been hypothesized as a novel drug target to resolve tuberculosis infections. The targeting of MgtC was a result of observing that upon deletion of the protein from ''M. tuberculosis'', the bacteria are no longer able to survive due to inhibition of [http://en.wiktionary.org/wiki/intramacrophage intramacrophage] growth. <ref name="mgtc">Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263.</ref> | + | [http://en.wikipedia.org/wiki/Tuberculosis Tuberculosis], caused by ''[[Mycobacterium tuberculosis]]'', is a respiratory infection still prevalent throughout the world. During the last decade, the emergence of [http://en.wikipedia.org/wiki/Multiple_drug_resistance multi-drug resistant] strains of ''M. tuberculosis'' has given rise to the need for the development of new antibiotics in order to combat the infection<ref>Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269. DOI: doi: 10.3109/1040841X.2010.482923.</ref>. In order to develop an efficacious antibiotic, the drug must be able to target a unique aspect of the bacteria, such as a protein, that is critical for its full virulence and survival. MgtC, an [http://en.wikipedia.org/wiki/Integral_membrane_protein integral protein] embedded in the extracellular membrane of ''M. tuberculosis'', has recently been hypothesized as a novel drug target to resolve tuberculosis infections. The targeting of MgtC was a result of observing that upon deletion of the protein from ''M. tuberculosis'', the bacteria are no longer able to survive due to inhibition of [http://en.wiktionary.org/wiki/intramacrophage intramacrophage] growth. <ref name="mgtc">Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263. DOI: 10.1128/JB.01424-12.</ref>. |
== Structure == | == Structure == | ||
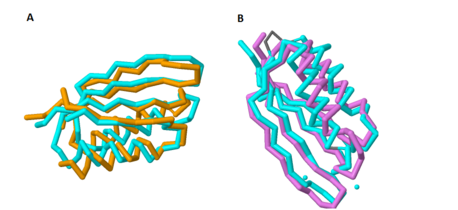
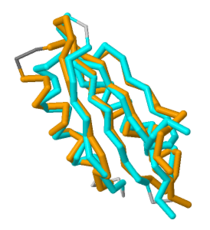
Based on its [http://en.wikipedia.org/wiki/Protein_tertiary_structure tertiary structure], this protein has been placed into a larger group of proteins known as the MgtC superfamily. The overall structure of MgtC is constituted by two [http://en.wikipedia.org/wiki/Protein_domain domains]: an N-terminal domain and a C-terminal domain. Each of these domains have striking similarities and differences with other MgtC-like proteins.<ref name="mgtc"/> | Based on its [http://en.wikipedia.org/wiki/Protein_tertiary_structure tertiary structure], this protein has been placed into a larger group of proteins known as the MgtC superfamily. The overall structure of MgtC is constituted by two [http://en.wikipedia.org/wiki/Protein_domain domains]: an N-terminal domain and a C-terminal domain. Each of these domains have striking similarities and differences with other MgtC-like proteins.<ref name="mgtc"/> | ||
| Line 83: | Line 83: | ||
===Role in Dimerization=== | ===Role in Dimerization=== | ||
| - | The potential for [http://en.wikipedia.org/wiki/Protein_dimer dimerization] was another aspect of MgtC which was studied to see if this protein forms complexes with proteins of known function. A [http://subtiwiki.uni-goettingen.de/wiki/index.php/BACTH Bacterial Two-Hybrid (BACTH)] assay was performed to study the potential for the entire protein to dimerize with itself and the potential for individual domains to dimerize. The results of this assay showed that the entire MgtC protein likely dimerizes, but the individual domains do not. This dimerization could serve as a critical component to the biochemical function of MgtC, although the exact implications have not yet been discerned <ref name="mgtc"/>. Frantz ''et al'' proposed a role for MgtC to form dimers with [http://proteopedia.org/wiki/index.php/2mc7 MgtR] (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=2MC7 2MC7]), a protein that serves to promote the degradation of MgtC. This has huge implications in the overall clinical relevance of how MgtC could be targeted to develop new-generation antibiotics. <ref name="mgtr">Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study.</ref> | + | The potential for [http://en.wikipedia.org/wiki/Protein_dimer dimerization] was another aspect of MgtC which was studied to see if this protein forms complexes with proteins of known function. A [http://subtiwiki.uni-goettingen.de/wiki/index.php/BACTH Bacterial Two-Hybrid (BACTH)] assay was performed to study the potential for the entire protein to dimerize with itself and the potential for individual domains to dimerize. The results of this assay showed that the entire MgtC protein likely dimerizes, but the individual domains do not. This dimerization could serve as a critical component to the biochemical function of MgtC, although the exact implications have not yet been discerned <ref name="mgtc"/>. Frantz ''et al'' proposed a role for MgtC to form dimers with [http://proteopedia.org/wiki/index.php/2mc7 MgtR] (PDB: [http://www.rcsb.org/pdb/explore/explore.do?structureId=2MC7 2MC7]), a protein that serves to promote the degradation of MgtC. This has huge implications in the overall clinical relevance of how MgtC could be targeted to develop new-generation antibiotics. <ref name="mgtr">Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study. DOI:10.1016/j.jmb.2013.10.014</ref> |
Revision as of 00:12, 11 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
MgtC: A Virulence Factor From Mycobacterium tuberculosis
| |||||||||||
References
- ↑ Singh, G.; Singh, G.; Jadeja, D.; Kaur, J. Lipid hydrolyzing enzymes in virulence: Mycobacterium tuberculosis as a model system. Critical Reviews in Microbiology 2010, 36(3): 259-269. DOI: doi: 10.3109/1040841X.2010.482923.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Yang, Y.; Labesse, G.; Carrere-Kremer, S.; Esteves, K.; Kremer, L.; Cohen-Gonsaud, M.; Blanc-Potard, A. The C-terminal domain of the virulence factor mgtc is a divergent act domain. J Bacteriol. 2012, 194(22): 6255-6263. DOI: 10.1128/JB.01424-12.
- ↑ 3.0 3.1 Jean-Francois, F.L.; Dai, J.; Yu, L. ; Myrick, A. ; Rubin, E. ; et al. Binding of mgtr, a salmonella transmembrane regulatory peptide, to mgtc, a mycobacterium tuberculosis virulence factor: a structural study. DOI:10.1016/j.jmb.2013.10.014