We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1068
From Proteopedia
(Difference between revisions)
| Line 16: | Line 16: | ||
[[Image:Active_site_cleft.png|300 px|left|thumb|Figure 2: This shows a single sub unit of MbtI, with the active site cleft located at the lower left hand side of the image.]] | [[Image:Active_site_cleft.png|300 px|left|thumb|Figure 2: This shows a single sub unit of MbtI, with the active site cleft located at the lower left hand side of the image.]] | ||
The crystal asymmetric unit was found to contain <scene name='69/694235/3log/1'> four MbtI molecules</scene>, however crystal packing and size exclusion chromatography data suggest a monomeric enzyme. There are no significant structural changes between the four monomers excepts from the localized differences in the active site. The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes. The core of the protein is formed by 21 <scene name='69/694235/Beta_sheets/3'>beta-strands</scene> folded into a twisted beta-sandwich. The protein's core is then surrounded by 10 alpha helices(Figure 2). | The crystal asymmetric unit was found to contain <scene name='69/694235/3log/1'> four MbtI molecules</scene>, however crystal packing and size exclusion chromatography data suggest a monomeric enzyme. There are no significant structural changes between the four monomers excepts from the localized differences in the active site. The overall molecular structure consist of a polypeptide of 450 residues that forms one large single domain with a similar fold to other chromate-utilizing enzymes. The core of the protein is formed by 21 <scene name='69/694235/Beta_sheets/3'>beta-strands</scene> folded into a twisted beta-sandwich. The protein's core is then surrounded by 10 alpha helices(Figure 2). | ||
| - | |||
| - | <scene name='69/694235/Almost_done/1'>transparency setting is not working</scene> | ||
| - | |||
== Disease == | == Disease == | ||
| - | |||
''Mycobacterium tuberculosis'' is the causative agent of Tuberculosis (TB), an infectious disease that affects one-third of the worlds population. Two TB-related conditions exist: latent TB infection and active TB disease. Currently, there are four regimens that are approved for the treatment of latent TB infection through the use of the antibiotics isoniazid, rifampin, and rifapentine.TB disease can also be treated through various antibiotic regimens. There are 10 drugs currently approved by the FDA for treating TB disease. The first-line anti-TB agents are the antibiotics isoniazid, rifampin, ethambutol, and pyrazinamide <ref>Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.</ref>. Although various treatments for TB infection and TB disease exist, the emergence of multi-drug and extensively-drug resistant strains of ''M. tuberculosis'' has increased the need for anti-tubercular agents with novel modes of action. | ''Mycobacterium tuberculosis'' is the causative agent of Tuberculosis (TB), an infectious disease that affects one-third of the worlds population. Two TB-related conditions exist: latent TB infection and active TB disease. Currently, there are four regimens that are approved for the treatment of latent TB infection through the use of the antibiotics isoniazid, rifampin, and rifapentine.TB disease can also be treated through various antibiotic regimens. There are 10 drugs currently approved by the FDA for treating TB disease. The first-line anti-TB agents are the antibiotics isoniazid, rifampin, ethambutol, and pyrazinamide <ref>Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.</ref>. Although various treatments for TB infection and TB disease exist, the emergence of multi-drug and extensively-drug resistant strains of ''M. tuberculosis'' has increased the need for anti-tubercular agents with novel modes of action. | ||
Revision as of 05:49, 11 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
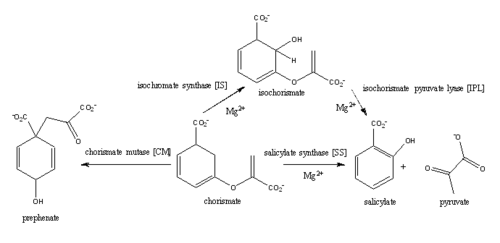
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Manos-Turvey A, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem. 2012 Dec 14;10(46):9223-36. doi: 10.1039/c2ob26736e. Epub 2012, Oct 29. PMID:23108268 doi:http://dx.doi.org/10.1039/c2ob26736e
- ↑ Chi G, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. Implications of Binding Mode and Active Site Flexibility for Inhibitor Potency against the Salicylate Synthase from Mycobacterium tuberculosis. Biochemistry. 2012 Jun 7. PMID:22607697 doi:10.1021/bi3002067
- ↑ Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.