We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1063
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='3R44' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='3R44' size='340' side='right' caption='Caption for this structure' scene=''> | ||
== Introduction == | == Introduction == | ||
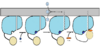
| - | ''<scene name='69/694230/Fadd13_subunits/3'>FadD13</scene>'' ''Mycobacterium Tuberculosis'' is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein. ACS proteins activate [http://en.wikipedia.org/wiki/Lipid | + | ''<scene name='69/694230/Fadd13_subunits/3'>FadD13</scene>'' ''Mycobacterium Tuberculosis'' is an ACSVL (Acyl-CoA synthetases very long) peripheral membrane protein. ACS proteins activate [http://en.wikipedia.org/wiki/Lipid lipids] and [http://en.wikipedia.org/wiki/Fatty_acid fatty acids] before going into metabolic pathways. FadD13 is soluble unlike other ACSVL proteins. FadD13 contains a hydrophobic tunnel for fatty acids to bind to, as well as an arginine rich lid loop that binds to the cell membrane. The binding of ATP causes structural changes promoting the binding of the hydrophobic substrates. Formation of an acyl-adenylate intermediate induces a 140 degree rotation of the small domain and binding of CoA for production of the final product, a fatty acyl-CoA thioester. Shown below is the general mechanism for ACS proteins. |
[[Image:FadD13 steps.jpg|350 px|thumb|left|Mechanism]] | [[Image:FadD13 steps.jpg|350 px|thumb|left|Mechanism]] | ||
Revision as of 12:21, 14 April 2015
FadD13
| |||||||||||