Sandbox Reserved 1074
From Proteopedia
| Line 13: | Line 13: | ||
[http://en.wikipedia.org/wiki/Mycolic_acid Mycolic acids] are very long-chain fatty acids (C<sub>60</sub> -C<sub>90</sub>) that are essential components of the mycobacterial cell wall. Mycolic acids are synthesized by at least two known elongation systems, type I and type II [http://en.wikipedia.org/wiki/Fatty_acid_synthase fatty acid synthases] (FAS-I and FAS-II) <ref name="FAS-II"> Bhatt, A. ''et al.'' (2007). The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. ''Journal of Molecular Microbiology, 64(6),'' 1442-1454. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/17555433 17555433] DOI: [http://www.ncbi.nlm.nih.gov/pubmed/17555433 10.1111/j.1365-2958.2007.05761.x]</ref>. The FAS-II system prefers C16 as a starting substrate and can extend up to C56 <ref name="FAS-II system"> Marrakchi, Hedia, ''et al.'' (2000). InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. ''Journal of Microbiology, 146,'' 289-296. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/10708367 10708367] </ref>. The FAS-II system utilizes the products from the FAS-I system as primers to extend the chain lengths further. The products of the FAS-II system are the precursors of mycolic acids. Elongation by the FAS-II system occurs by a [http://en.wikipedia.org/wiki/Condensation_reaction condensation reaction]of [http://en.wikipedia.org/wiki/Acetyl acetyl] and [http://en.wikipedia.org/wiki/Malonyl-CoA malonyl] substrates, which is achieved in three steps. Step 1 involves transfer of the acyl primer, step 2 involves [http://en.wikipedia.org/wiki/Decarboxylation decarboxylation] of the substrate to yield a [http://en.wikipedia.org/wiki/Carbanion carbanion], and step 3 involves nucleophilic attack of the carbanion to yield the elongated product <ref name="FAS-II" />. | [http://en.wikipedia.org/wiki/Mycolic_acid Mycolic acids] are very long-chain fatty acids (C<sub>60</sub> -C<sub>90</sub>) that are essential components of the mycobacterial cell wall. Mycolic acids are synthesized by at least two known elongation systems, type I and type II [http://en.wikipedia.org/wiki/Fatty_acid_synthase fatty acid synthases] (FAS-I and FAS-II) <ref name="FAS-II"> Bhatt, A. ''et al.'' (2007). The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. ''Journal of Molecular Microbiology, 64(6),'' 1442-1454. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/17555433 17555433] DOI: [http://www.ncbi.nlm.nih.gov/pubmed/17555433 10.1111/j.1365-2958.2007.05761.x]</ref>. The FAS-II system prefers C16 as a starting substrate and can extend up to C56 <ref name="FAS-II system"> Marrakchi, Hedia, ''et al.'' (2000). InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. ''Journal of Microbiology, 146,'' 289-296. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/10708367 10708367] </ref>. The FAS-II system utilizes the products from the FAS-I system as primers to extend the chain lengths further. The products of the FAS-II system are the precursors of mycolic acids. Elongation by the FAS-II system occurs by a [http://en.wikipedia.org/wiki/Condensation_reaction condensation reaction]of [http://en.wikipedia.org/wiki/Acetyl acetyl] and [http://en.wikipedia.org/wiki/Malonyl-CoA malonyl] substrates, which is achieved in three steps. Step 1 involves transfer of the acyl primer, step 2 involves [http://en.wikipedia.org/wiki/Decarboxylation decarboxylation] of the substrate to yield a [http://en.wikipedia.org/wiki/Carbanion carbanion], and step 3 involves nucleophilic attack of the carbanion to yield the elongated product <ref name="FAS-II" />. | ||
| - | === ''' | + | === '''Reaction Catalyzed''' === |
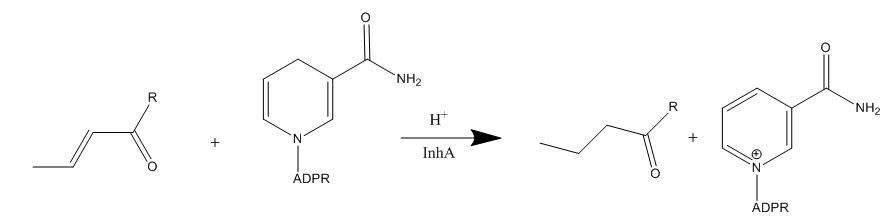
The InhA gene encodes for the InhA protein. InhA catalyzes the NADH-dependent reduction of the ''trans'' double bond between positions C2-C3 of fatty acyl substrates. InhA prefers fatty acyl substrates of C16 or longer, which is consistent of the protein being a member of the FAS-II system. The longer chain length specificity of InhA distinguishes the enzyme from other enoyl-ACP reductase analogues. | The InhA gene encodes for the InhA protein. InhA catalyzes the NADH-dependent reduction of the ''trans'' double bond between positions C2-C3 of fatty acyl substrates. InhA prefers fatty acyl substrates of C16 or longer, which is consistent of the protein being a member of the FAS-II system. The longer chain length specificity of InhA distinguishes the enzyme from other enoyl-ACP reductase analogues. | ||
| - | [[Image:Mechanism-InhA.JPG|thumb|1000px|center|Figure 1. | + | [[Image:Mechanism-InhA.JPG|thumb|1000px|center|Figure 1. Reaction catalyzed by InhA protein <ref name="InhA"/> ]] |
Revision as of 19:39, 15 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Contents |
Enoyl-ACP Reductase InhA from Mycobacterium tuberculosis
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Bell, A.F. et al.(2007). Evidence from Raman Spectroscopy That InhA , the Mycobacterial Enoyl Reductase, Modulates the Conformation of the NADH Cofactor to Promote Catalysis. Journal of the American Chemical Society, 129, 6425-6431. DOI: 10.1021/ja068219m
- ↑ 2.0 2.1 Bhatt, A. et al. (2007). The Mycobacterium tuberculosis FAS-II condensing enzymes: their role in mycolic acid biosynthesis, acid-fastness, pathogenesis and in future drug development. Journal of Molecular Microbiology, 64(6), 1442-1454. PMID: 17555433 DOI: 10.1111/j.1365-2958.2007.05761.x
- ↑ Marrakchi, Hedia, et al. (2000). InhA, a target of the antituberculous drug isoniazid, is involved in a mycobacterial fatty acid elongation system, FAS-II. Journal of Microbiology, 146, 289-296. PMID: 10708367
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 Rozwarski, D.A. et al. (1999). Crystal Structure of the Mycobacterium tuberculosis Enoyl-ACP Reductase, InhA, in Complex with NAD+ and a C16 Fatty Acyl Substrate. Journal of Biological Chemistry, 274(22), 15582-15589. PMID: 10336454 DOI: 10.1074/jbc.274.22.15582
- ↑ 5.0 5.1 5.2 Vilchèze, C. et al. (2011). Novel Inhibitors of InhA Efficiently Kill Mycobacterium tuberculosis under Aerobic and Anaerobic Conditions. Antimicrobial Agents and Chemotherapy, 55(8), 3889-3898. DOI: 10.1128/AAC.00266-11
Student Contributors
- Arielle Russell
- Mackenzie A. Smith
Similar Proteopedia Pages
Enoyl-Acyl-Carrier Protein Reductase
Additional 3D Structures of Enoyl-ACP Reductase InhA
3oew, 2x22, 2x23, 1eny, 1enz, 4dqu, 4dre - MtENR+NAD; 3of2, 4dti - MtENR(mutant)+NAD; 2pr2, 2idz, 2h9i - MtENR+INH-NAPD; 2aq8 - MtENR+NADH; 2aqh, 2aqi, 2aqk, 3oey - MtENR(mutant)+NADH; 2ntj - MtENR+PTH-NAD; 2ie0, 2ieb, 2nv6, 1zid - MtENR(mutant)+INH-NAPD; 3fne, 3fnf, 3fng, 3fnh, 2b35, 1p45 - MtENR+NAD+TCI; 2b36, 2b37, 4ohu, 4oim, 4oyr - MtENR+NAD+phenoxyphenol derivative; 2nsd - MtENR+NAD+piperidine derivative; 2h7l, 2h7m, 2h7n, 2h7p, 4u0j, 4tzt, 4tzk, 4trj, 4u0k - MtENR+NAD+pyrrolidine derivative; 4cod, 4bqp, 4bqr, 4bge, 4bii, 4oxk, 4oxn, 4oxy, 4r9r, 4r9s - MtENR+NAD + inhibitor; 4bgi - MtENR (mutant)+NAD+inhibitor; 1p44 - MtENR+NAD+indole derivative; 1bvr - MtENR+NAD+fatty-acyl substrate