We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1075
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
== General Structure and Function == | == General Structure and Function == | ||
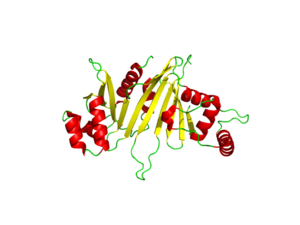
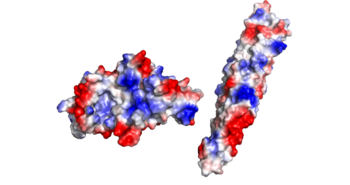
| - | The crystal structure of EspG shows the protein in solution. As a monomeric protein, this binds to its ligand with high specificity. The EspG3 protein shown has a mass of 33.7kD <ref>PMID:25275011</ref> . The proteins beta sheets make up the backbone of the protein (yellow). This backbone includes the key residues that interact with the random coil of the ligand. This is surrounded by 8 alpha helices (red) which add to the structure of the protein. One key <scene name='69/694242/Espg3_differences/2'>alpha helix</scene> will vary per EspG protein to stericly limit binding to PE-PPE ligand. The random coil (green) connects the beta sheets and helices together, where the long <scene name='69/694242/Espg3_differences/3'>random loop</scene> variations can impact binding to the EspG's ligand. | + | The crystal structure of EspG shows the protein in solution. As a monomeric protein, this binds to its ligand with high specificity. The EspG3 protein shown has a mass of 33.7kD <ref>PMID:25275011</ref> . The proteins beta sheets make up the backbone of the protein (yellow). A key beta sheet region on the β-2, β-3 will vary between EspG proteins to influence what the random loop on the PE-PPE protein will interact with. This backbone includes the key residues that interact with the random coil of the ligand. This is surrounded by 8 alpha helices (red) which add to the structure of the protein. One key <scene name='69/694242/Espg3_differences/2'>alpha helix</scene> will vary per EspG protein to stericly limit binding to PE-PPE ligand. The random coil (green) connects the beta sheets and helices together, where the long <scene name='69/694242/Espg3_differences/3'>random loop</scene> variations can impact binding to the EspG's ligand. |
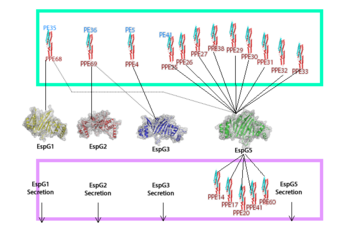
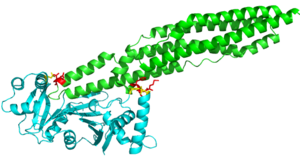
Through specific binding factors, an EspG binds to its PE-PPE ligand to be secreted through the ESAT pathway. Though the ESAT-6 secretion system is poorly understood, it is known that PE-PPE proteins and EspG proteins influence virulence and pathogenicity of the infection. | Through specific binding factors, an EspG binds to its PE-PPE ligand to be secreted through the ESAT pathway. Though the ESAT-6 secretion system is poorly understood, it is known that PE-PPE proteins and EspG proteins influence virulence and pathogenicity of the infection. | ||
Revision as of 00:13, 16 April 2015
| |||||||||||
References
- ↑ Ekiert DC, Cox JS. Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14758-63. doi:, 10.1073/pnas.1409345111. Epub 2014 Oct 1. PMID:25275011 doi:http://dx.doi.org/10.1073/pnas.1409345111
- ↑ Renshaw PS, Lightbody KL, Veverka V, Muskett FW, Kelly G, Frenkiel TA, Gordon SV, Hewinson RG, Burke B, Norman J, Williamson RA, Carr MD. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J. 2005 Jul 20;24(14):2491-8. Epub 2005 Jun 23. PMID:15973432
- ↑ Ekiert DC, Cox JS. Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14758-63. doi:, 10.1073/pnas.1409345111. Epub 2014 Oct 1. PMID:25275011 doi:http://dx.doi.org/10.1073/pnas.1409345111
Similar Pages
Student Contributors
- Mark Meredith
- Jonathan Golliher