Sandbox Reserved 1075
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
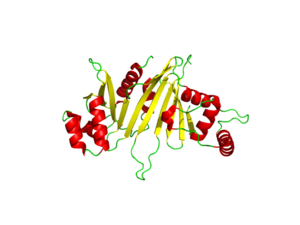
[[Image:EspG_PPE-PE_secretion.fw_(2).png|350 px|left|thumb|[http://en.wikipedia.org/wiki/CFP-10 "Specificity of EspG Binding"]]] | [[Image:EspG_PPE-PE_secretion.fw_(2).png|350 px|left|thumb|[http://en.wikipedia.org/wiki/CFP-10 "Specificity of EspG Binding"]]] | ||
| - | |||
| - | |||
| - | |||
| Line 27: | Line 24: | ||
== Binding == | == Binding == | ||
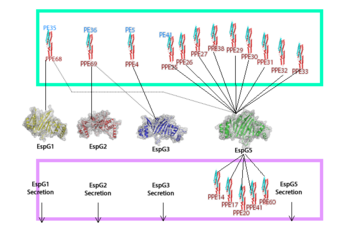
The EspG-PE-PPE binding is highly specific. The specific pair we were looking at was the EspG5-PE25-PPE41 complex. A variety of binding factors influence the EspG that will bind to a specific PE-PPE. The secretion pathway carried out needs the coupled protein. | The EspG-PE-PPE binding is highly specific. The specific pair we were looking at was the EspG5-PE25-PPE41 complex. A variety of binding factors influence the EspG that will bind to a specific PE-PPE. The secretion pathway carried out needs the coupled protein. | ||
| - | |||
| - | |||
'''Residue Interactions:''' | '''Residue Interactions:''' | ||
<scene name='69/694242/Specific_contact_residues/1'>Random Loop, β2-β3 interactions</scene> | <scene name='69/694242/Specific_contact_residues/1'>Random Loop, β2-β3 interactions</scene> | ||
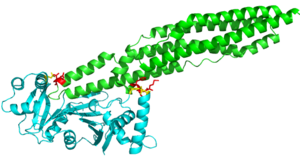
| - | The random loop on the cigar shaped PE-PPE ligand binds to the β2-β3 sheets on this EspG protein. | + | The random loop on the cigar shaped PE-PPE ligand binds to the β2-β3 sheets on this EspG protein. The residues on the random turn in the PE-PPE protein are key for the specificity to the EspG protein. Coils between PE-PPE proteins vary greatly and influence binding affinity. Combined with its β-2 & β-3 interactions on the EspG protein, the EspG protein is PE-PPE specific. These make up the bulk of residue interactions in the complex. An EspG protein will not bind to its non-corresponding PE-PPE complex. The residue hinderance image shows the hypothetical consequences of a non-corresponding EspG-PE-PPE bound complex. Here we see specific amino acid residue contact hinderance between the Asp36 and Gln172 on the EspG, with Asn122, His2, Phe3 and Met1 on the PE25-PPE41 complex. |
| + | |||
| + | [[Image:EspG3_PPE_hinderance_W.png|300 px|left|thumb|Residue Hinderance]] | ||
| - | The residues on the random turn in the PE-PPE protein are key for the specificity to the EspG protein. Coils between PE-PPE proteins vary greatly and influence binding affinity. Combined with its β-2 & β-3 interactions on the EspG protein, the EspG protein is PE-PPE specific. These make up the bulk of residue interactions in the complex. | ||
| - | [[Image:EspG3_PPE_hinderance_W.png|300 px|left|thumb|Residue Hinderance]] | ||
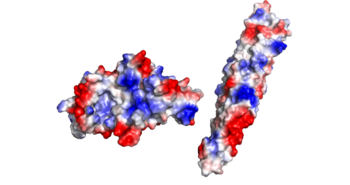
<scene name='69/694242/Glutamate_interractions/3'>Dipole-Dipole Glutamate Interactions</scene> | <scene name='69/694242/Glutamate_interractions/3'>Dipole-Dipole Glutamate Interactions</scene> | ||
Revision as of 00:31, 16 April 2015
| |||||||||||
References
- ↑ Ekiert DC, Cox JS. Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14758-63. doi:, 10.1073/pnas.1409345111. Epub 2014 Oct 1. PMID:25275011 doi:http://dx.doi.org/10.1073/pnas.1409345111
- ↑ Renshaw PS, Lightbody KL, Veverka V, Muskett FW, Kelly G, Frenkiel TA, Gordon SV, Hewinson RG, Burke B, Norman J, Williamson RA, Carr MD. Structure and function of the complex formed by the tuberculosis virulence factors CFP-10 and ESAT-6. EMBO J. 2005 Jul 20;24(14):2491-8. Epub 2005 Jun 23. PMID:15973432
- ↑ Ekiert DC, Cox JS. Structure of a PE-PPE-EspG complex from Mycobacterium tuberculosis reveals molecular specificity of ESX protein secretion. Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14758-63. doi:, 10.1073/pnas.1409345111. Epub 2014 Oct 1. PMID:25275011 doi:http://dx.doi.org/10.1073/pnas.1409345111
Similar Pages
Student Contributors
- Mark Meredith
- Jonathan Golliher