We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1066
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
''Mycobacterium tuberculosis'' very-long-chain fatty acyl-CoA synthetase, also known as FadD13, is unique within its class of FadD proteins in regards to its ability to house lipid substrates longer than itself. These lipid substrates are very-long-chain fatty acids between lengths C22 –C26, which is up to the maximum length tested. <ref name="Our Paper"/> The significance of theses very-long-chain fatty acids lies in their importance to mycolic acid synthesis by ''Mycobacterium tuberculosis'' ''(M. tb)''. Mycolic acids compose part of the cell wall of ''(M. tb)''. FadD13, an activator of mycolic acids, has been identified as key component in the virulence of [https://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''Mycobacterium tuberculosis''], the etiological agent of [https://en.wikipedia.org/wiki/Tuberculosis tuberculosis], and has emerged as possible target for novel therapeutic agents.<ref name="JT">PMID: 20454815</ref> The FadD13 enzyme is the last gene of the ''mymA'' operon. <ref name="residue paper"/> | ''Mycobacterium tuberculosis'' very-long-chain fatty acyl-CoA synthetase, also known as FadD13, is unique within its class of FadD proteins in regards to its ability to house lipid substrates longer than itself. These lipid substrates are very-long-chain fatty acids between lengths C22 –C26, which is up to the maximum length tested. <ref name="Our Paper"/> The significance of theses very-long-chain fatty acids lies in their importance to mycolic acid synthesis by ''Mycobacterium tuberculosis'' ''(M. tb)''. Mycolic acids compose part of the cell wall of ''(M. tb)''. FadD13, an activator of mycolic acids, has been identified as key component in the virulence of [https://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''Mycobacterium tuberculosis''], the etiological agent of [https://en.wikipedia.org/wiki/Tuberculosis tuberculosis], and has emerged as possible target for novel therapeutic agents.<ref name="JT">PMID: 20454815</ref> The FadD13 enzyme is the last gene of the ''mymA'' operon. <ref name="residue paper"/> | ||
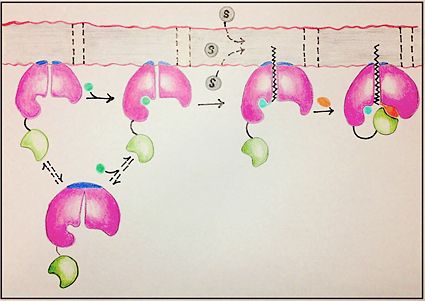
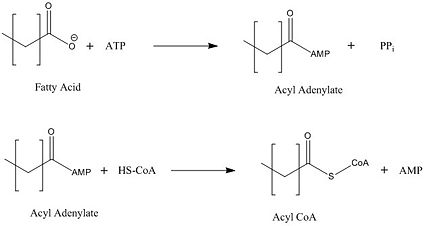
| - | There are four main groups of FadD enzymes categorized on their ability to accommodate different length substrates: short (C2-C4), medium (C4-C12), long (C12-C22), and very long (C22-C26).<ref name="Our Paper"/> Most FadD class proteins exist as integral membrane proteins, involved in both the activation of fatty acids and other hydrophobic substrates in addition to the transport of these lipids into the cell. However, substrates longer than the enzyme itself, like these very-long-chain fatty acids, pose | + | There are four main groups of FadD enzymes categorized on their ability to accommodate different length substrates: short (C2-C4), medium (C4-C12), long (C12-C22), and very long (C22-C26).<ref name="Our Paper"/> Most FadD class proteins exist as integral membrane proteins, involved in both the activation of fatty acids and other hydrophobic substrates in addition to the transport of these lipids into the cell. However, substrates longer than the enzyme itself, like these very-long-chain fatty acids, pose an interesting structural dilemma to the enzyme. FadD13 differs from typical integral membrane fatty acyl-CoA synthetases in that FadD13 exists as a peripheral membrane protein. This key feature provides a mechanistic basis for FadD13’s activation and transport of fatty acids of length C24-C26 through the two step addition of [http://en.wikipedia.org/wiki/Coenzyme_A Coenzyme A](Figure 1).<ref name="Our Paper">PMID: 22560731</ref> |
| Line 26: | Line 26: | ||
=Structure = | =Structure = | ||
| - | FadD13 is composed of 503 amino acid residues divided into three main regions: The <scene name='69/694233/N_terminal_domain/1'>N-terminal domain</scene> (residues 1-395) and <scene name='69/694233/C-terminal_domain/2'>C-terminal domain</scene> (residues 402-503) which are connected via a flexible <scene name='69/694233/Linker_section/2'>linker</scene> represented in dark blue (residues 396-401).<ref name="Our Paper"/> Each region plays an important role in the activation of fatty acids. The large N-terminal domain houses | + | FadD13 is composed of 503 amino acid residues divided into three main regions: The <scene name='69/694233/N_terminal_domain/1'>N-terminal domain</scene> (residues 1-395) and <scene name='69/694233/C-terminal_domain/2'>C-terminal domain</scene> (residues 402-503) which are connected via a flexible <scene name='69/694233/Linker_section/2'>linker</scene> represented in dark blue (residues 396-401).<ref name="Our Paper"/> Each region plays an important role in the activation of fatty acids. The large N-terminal domain houses many key structural features involved in fatty acid activation, but ultimately it is the flexible linker that allows movement of the C-terminal domain to from the fully functioning active site of FadD13 (Figure 1). |
| Line 38: | Line 38: | ||
== Hydrophobic Tunnel == | == Hydrophobic Tunnel == | ||
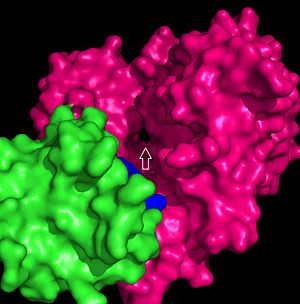
| - | The hydrophobic tunnel of FadD13 is essential to the transport and accommodation of very long fatty acids from the membrane into the cell. This tunnel runs through the middle of FadD13 from the arginine rich lid loop to the ATP binding site and is situated between the and alpha helices α8-α9 and parallel beta sheet β9- β14 (Figure | + | The hydrophobic tunnel of FadD13 is essential to the transport and accommodation of very long fatty acids from the membrane into the cell. This tunnel runs through the middle of FadD13 from the arginine rich lid loop to the ATP binding site and is situated between the and alpha helices α8-α9 and parallel beta sheet β9- β14 (Figure 4).<ref name="Our Paper"/> Negatively charged residues at the active site of FadD13 are the driving factor in the attraction of the fatty acid from the membrane through the hydrophobic tunnel of the enzyme. |
[[Image:Hydrophobic tunnel 2.jpg|300 px|left|thumb|Figure 4: Pmyol depiction of hydrophobic tunnel.]] | [[Image:Hydrophobic tunnel 2.jpg|300 px|left|thumb|Figure 4: Pmyol depiction of hydrophobic tunnel.]] | ||
| Line 44: | Line 44: | ||
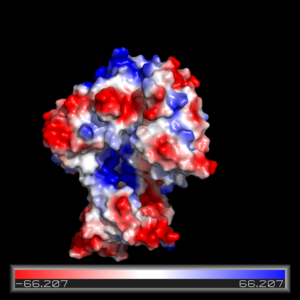
The FadD13 active site is composed of positively charged regions which account for the attraction and binding of hydrophobic substrates to this region (Figure 3). The active site on FadD13 is composed of two conserved regions, one of which serves as the binding site for ATP and the other for CoA. The adenine of ATP is bound to a group of <scene name='69/694232/Adenine_binding_group/2'>six amino acids (300-305)</scene> that is structurally identically to other acyl-CoA synthetases. <ref name="Our Paper"/> | The FadD13 active site is composed of positively charged regions which account for the attraction and binding of hydrophobic substrates to this region (Figure 3). The active site on FadD13 is composed of two conserved regions, one of which serves as the binding site for ATP and the other for CoA. The adenine of ATP is bound to a group of <scene name='69/694232/Adenine_binding_group/2'>six amino acids (300-305)</scene> that is structurally identically to other acyl-CoA synthetases. <ref name="Our Paper"/> | ||
| - | + | Mutation of the highly conserved residue in the C-terminal region, <scene name='69/694233/Lys_487/2'>Lysine 487</scene>, resulted in a 95% loss of function of FadD13 and is thought to be involved in the orientation of the substrates to form the adenylate intermediate.<ref name="residue paper">PMID: 20027301</ref> Additionally, Serine 404 was hypothesized to be involved in the binding of Coenzyme A which may only occur once this region incurs a 140 degree rotational change after the initial binding of ATP.<ref name="Our Paper"/><ref name="residue paper"/> | |
Revision as of 17:26, 17 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Mycobacterium tuberculosis very-long-chain fatty acyl-CoA synthetase
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 Andersson CS, Lundgren CA, Magnusdottir A, Ge C, Wieslander A, Molina DM, Hogbom M. The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: Structural Basis for Housing Lipid Substrates Longer than the Enzyme. Structure. 2012 May 2. PMID:22560731 doi:10.1016/j.str.2012.03.012
- ↑ Jatana N, Jangid S, Khare G, Tyagi AK, Latha N. Molecular modeling studies of Fatty acyl-CoA synthetase (FadD13) from Mycobacterium tuberculosis--a potential target for the development of antitubercular drugs. J Mol Model. 2011 Feb;17(2):301-13. doi: 10.1007/s00894-010-0727-3. Epub 2010 May, 8. PMID:20454815 doi:http://dx.doi.org/10.1007/s00894-010-0727-3
- ↑ 3.0 3.1 3.2 3.3 3.4 Khare G, Gupta V, Gupta RK, Gupta R, Bhat R, Tyagi AK. Dissecting the role of critical residues and substrate preference of a Fatty Acyl-CoA Synthetase (FadD13) of Mycobacterium tuberculosis. PLoS One. 2009 Dec 21;4(12):e8387. doi: 10.1371/journal.pone.0008387. PMID:20027301 doi:10.1371/journal.pone.0008387
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Jatana N, Jangid S, Khare G, Tyagi AK, Latha N. Molecular modeling studies of Fatty acyl-CoA synthetase (FadD13) from Mycobacterium tuberculosis--a potential target for the development of antitubercular drugs. J Mol Model. 2011 Feb;17(2):301-13. doi: 10.1007/s00894-010-0727-3. Epub 2010 May, 8. PMID:20454815 doi:http://dx.doi.org/10.1007/s00894-010-0727-3
- ↑ 5.0 5.1 5.2 Schroeder EK, de Souza N, Santos DS, Blanchard JS, Basso LA. Drugs that inhibit mycolic acid biosynthesis in Mycobacterium tuberculosis. Curr Pharm Biotechnol. 2002 Sep;3(3):197-225. PMID:12164478
External Resources
Tuberculosis Wikipedia page
Mycobacterium tuberculosis Wikipedia page
Coenzyme A Wikipedia page
Acyl CoA Wikipedia Page
Mycolic Acid Wikipedia page