This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1056
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structure == | == Structure == | ||
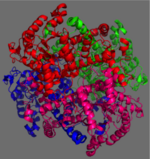
[[Image:homotetramer.png|150 px|left|thumb|Figure 2: 222 Symmetry of the homotetramer isocitrate lyase. Each identical monomer is shown in a unique color.]] | [[Image:homotetramer.png|150 px|left|thumb|Figure 2: 222 Symmetry of the homotetramer isocitrate lyase. Each identical monomer is shown in a unique color.]] | ||
| - | The ICL homotetramer possesses 222 symmetry, with an axis of rotation at x-axis, y-axis, and z-axis of the enzyme. Two individual subunits of ICL are held together by a characteristic <scene name='69/697526/Helix_swapping/3'>Helix Swapping</scene> between three alpha helices formed by residues 370-384, 349-367, and 399-409 on neighboring monomers<ref name="ICL">PMID:10932251</ref>. The interlocking mechanism created by these helices provides additional strength to hold the two monomeric subunits together, allowing ICL to be composed of a dimer of dimers<ref name="ICL2"/>. This interaction will bury approximately 18% of the surface of each subunit, and will help to shield the interior binding site from hydration. | + | The ICL homotetramer possesses 222 symmetry, with an axis of rotation at x-axis, y-axis, and z-axis of the enzyme. Two individual subunits of ICL, shown here as blue and green, are held together by a characteristic <scene name='69/697526/Helix_swapping/3'>Helix Swapping</scene> between three alpha helices formed by residues 370-384, 349-367, and 399-409 on neighboring monomers<ref name="ICL">PMID:10932251</ref>. The interlocking mechanism created by these helices provides additional strength to hold the two monomeric subunits together, allowing ICL to be composed of a dimer of dimers<ref name="ICL2"/>. This interaction will bury approximately 18% of the surface of each subunit, and will help to shield the interior binding site from hydration. |
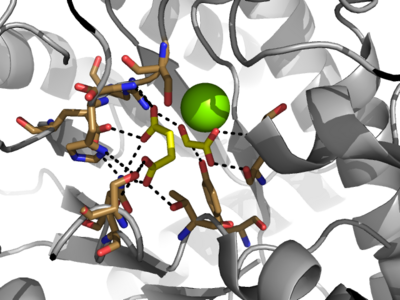
== Active Site == | == Active Site == | ||
Revision as of 18:29, 19 April 2015
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||
3D Structures of Isocitrate Lyase
Updated on 19-April-2015
- ICL from other bacteria
References
- ↑ Srivastava V, Jain A, Srivastava BS, Srivastava R. Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. Tuberculosis (Edinb). 2008 May;88(3):171-7. Epub 2007 Dec 3. PMID:18054522 doi:http://dx.doi.org/10.1016/j.tube.2007.10.002
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Sharma V, Sharma S, Hoener zu Bentrup K, McKinney JD, Russell DG, Jacobs WR Jr, Sacchettini JC. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat Struct Biol. 2000 Aug;7(8):663-8. PMID:10932251 doi:10.1038/77964
- ↑ 3.0 3.1 3.2 Beeching JR. High sequence conservation between isocitrate lyase from Escherichia coli and Ricinus communis. Protein Seq Data Anal. 1989 Dec;2(6):463-6. PMID:2696959
- ↑ 4.0 4.1 4.2 4.3 Masamune et al. Bio-Claisen condensation catalyzed by thiolase from Zoogloea ramigera. Active site cysteine residues. "Journal of the American Chemical Society" 111: 1879-1881 (1989). DOI: 10.1021/ja00187a053
- ↑ Connely, M. L. Solvent-accessible surfaces of proteins and nucleic acids "Science" 221:709-713 (1983). DOI: 10.1126/science.6879170