We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1072
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
==Structure== | ==Structure== | ||
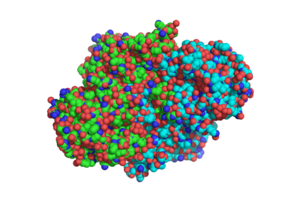
| - | [[Image:DotSTRUCTURE.png|300 px|left|thumb|Overall structure of [http://www.rcsb.org/pdb/explore/explore.do?structureId=1sJ2 1SJ2], showing that the structure is a [http://en.wikipedia.org/wiki/Protein_dimer homodimer].]] | + | [[Image:DotSTRUCTURE.png|300 px|left|thumb|'''Figure 1.'''Overall structure of [http://www.rcsb.org/pdb/explore/explore.do?structureId=1sJ2 1SJ2], showing that the structure is a [http://en.wikipedia.org/wiki/Protein_dimer homodimer].]] |
| Line 21: | Line 21: | ||
===Active Site=== | ===Active Site=== | ||
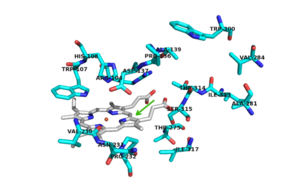
| - | [[Image:INH.png|300 px|left|thumb|Active Site]] | + | [[Image:INH.png|300 px|left|thumb|'''Figure 2.'''Active Site with conserved amino acid residues.]] |
---- | ---- | ||
| Line 34: | Line 34: | ||
==Clinical Applications== | ==Clinical Applications== | ||
===Isoniazid Structure=== | ===Isoniazid Structure=== | ||
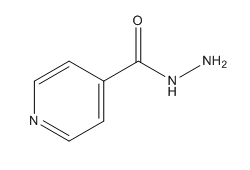
| - | [[Image:INH_structure._PNG.PNG|300 px|left|thumb|Chemical Structure of Isoniazid (INH)]] | + | [[Image:INH_structure._PNG.PNG|300 px|left|thumb|'''Figure 3.'''Chemical Structure of Isoniazid (INH)]] |
===Isoniazid Role=== | ===Isoniazid Role=== | ||
[http://en.wikipedia.org/wiki/Isoniazid INH] is a [http://en.wikipedia.org/wiki/Prodrug prodrug] susceptible to oxidative reactions catalyzed by KatG <ref name="four">PMID: 16566587</ref>. INH action against mycobacteria requires catalase-peroxidase (KatG) function. | [http://en.wikipedia.org/wiki/Isoniazid INH] is a [http://en.wikipedia.org/wiki/Prodrug prodrug] susceptible to oxidative reactions catalyzed by KatG <ref name="four">PMID: 16566587</ref>. INH action against mycobacteria requires catalase-peroxidase (KatG) function. | ||
| Line 40: | Line 40: | ||
===Formation of IN-NAD Adduct=== | ===Formation of IN-NAD Adduct=== | ||
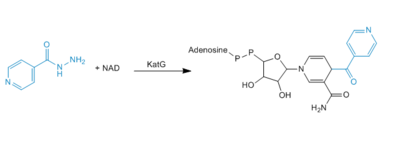
Activation or oxidation of INH by KatG has recently been measured in the terms of production of an IN-NAD adduct molecule that serves as a tight binding inhibitor of InhA. InhA is an enzyme involved in the biosynthesis of [http://en.wikipedia.org/wiki/Mycolic_acid mycolic acids], which are components of the mycobacterial cell wall. Therefore, inhibition of InhA alone is sufficient enough to inhibit mycolic acid biosynthesis and induce cell lysis after exposure of bacteria to INH (4). | Activation or oxidation of INH by KatG has recently been measured in the terms of production of an IN-NAD adduct molecule that serves as a tight binding inhibitor of InhA. InhA is an enzyme involved in the biosynthesis of [http://en.wikipedia.org/wiki/Mycolic_acid mycolic acids], which are components of the mycobacterial cell wall. Therefore, inhibition of InhA alone is sufficient enough to inhibit mycolic acid biosynthesis and induce cell lysis after exposure of bacteria to INH (4). | ||
| - | [[Image:INH_mechanism.PNG|400 px|right|thumb|Mechanism of adduct formation]] | + | [[Image:INH_mechanism.PNG|400 px|right|thumb|'''Figure 4.'''Mechanism of adduct formation]] |
| Line 47: | Line 47: | ||
Resistance to INH is a continuing problem in the development of effective therapeutic regimes designed to eliminate infections from ''M. tuberculosis''. Resistance to INH is due to deletions or mutations in this KatG catalase peroxidase enzyme. There are many possible mutations in this peroxidase that can play a role in the resistance of INH. The most commonly occurring mutation occurs at <scene name='69/694238/Active_site/7'>Ser 315</scene>. A mutation at this amino acid can result in up to a 200 fold increase in the minimum inhibitory concentration for INH. Ser 315 has been reported to mutate to asparagine, isoleucine, glycine, and most frequently, threonine. A S315T mutant has the ability to reduce the affinity of the enzyme for INH by increasing steric hindrance and reducing access to the substrate binding site <ref name="one"/>. Mutation of Ser315 to a Thr in ''mt''CP results in a loss of the activation to the anti-tuberculosis drug (INH) with no loss of either peroxidase or catalase activity (3). Any of the other mutations at this site, except for glycine, would also increase steric hindrance and decrease the accessibility to the binding site. | Resistance to INH is a continuing problem in the development of effective therapeutic regimes designed to eliminate infections from ''M. tuberculosis''. Resistance to INH is due to deletions or mutations in this KatG catalase peroxidase enzyme. There are many possible mutations in this peroxidase that can play a role in the resistance of INH. The most commonly occurring mutation occurs at <scene name='69/694238/Active_site/7'>Ser 315</scene>. A mutation at this amino acid can result in up to a 200 fold increase in the minimum inhibitory concentration for INH. Ser 315 has been reported to mutate to asparagine, isoleucine, glycine, and most frequently, threonine. A S315T mutant has the ability to reduce the affinity of the enzyme for INH by increasing steric hindrance and reducing access to the substrate binding site <ref name="one"/>. Mutation of Ser315 to a Thr in ''mt''CP results in a loss of the activation to the anti-tuberculosis drug (INH) with no loss of either peroxidase or catalase activity (3). Any of the other mutations at this site, except for glycine, would also increase steric hindrance and decrease the accessibility to the binding site. | ||
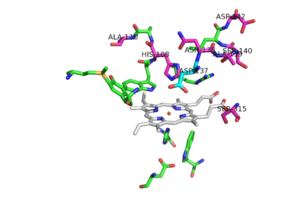
| - | [[Image:Mutation_locations.png|300 px|left|thumb|Pink residues represent the location of possible mutations. Green residues represent the active site. Asp 137 is shown in blue.]] | + | [[Image:Mutation_locations.png|300 px|left|thumb|'''Figure 5'''Pink residues represent the location of possible mutations. Green residues represent the active site. Asp 137 is shown in blue.]] |
Of the active site residues that are involved in enzyme catalyzed activation of INH, only <scene name='69/694238/Active_site/8'>His 108</scene> has been a site for mutations that can increase resistance to INH. His 108 has been reported to mutate to glutamic acid and [http://en.wikipedia.org/wiki/Glutamine glutamine]. These mutations reduce the affinity for INH but the hydrogen bond donor/acceptor groups of glutamine would still allow INH to bind. However, glutamine wouldn't be able to act as proton shuttle in the way His 108 does in the enzyme-catalyzed activation pathway. | Of the active site residues that are involved in enzyme catalyzed activation of INH, only <scene name='69/694238/Active_site/8'>His 108</scene> has been a site for mutations that can increase resistance to INH. His 108 has been reported to mutate to glutamic acid and [http://en.wikipedia.org/wiki/Glutamine glutamine]. These mutations reduce the affinity for INH but the hydrogen bond donor/acceptor groups of glutamine would still allow INH to bind. However, glutamine wouldn't be able to act as proton shuttle in the way His 108 does in the enzyme-catalyzed activation pathway. | ||
Revision as of 13:06, 21 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
| |||||||||||