This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1063
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Structural Highlights == | == Structural Highlights == | ||
| - | FadD13 is an ACSVL enzyme that can accept lipids up to 26 carbons as well as being a [http://en.wikipedia.org/wiki/Peripheral_membrane_protein peripheral-membrane protein]<ref>PMID: 20027301</ref>. Unlike other ACSVL proteins, FadD13 is soluble. There are numerous aspects of its structure that affects the way this protein functions. The <scene name='69/694230/Fadd13_subunits/12'>ATP/AMP binding region</scene> is comprised of two separate motifs. The first motif is composed of residues 164-TSGTTGHPKG173-173 shown in red which binds to the phosphate group. The second motif is comprised of residues 298-VQGYALTE-305 shown in blue which binds to the adenine group<ref>DOI: 10.1371/journal.pone.0008387</ref>. Upon binding of ATP or AMP, FadD13 is activated. There are two domains of this protein, a larger N-terminal domain (<scene name='69/694230/Fadd13_subunits/8'>residues 1-395</scene>) shown in blue and a smaller C-terminal domain (<scene name='69/694230/Fadd13_subunits/10'>residues 402-503</scene>) shown in yellow. These domains are held together by a six amino acid linker (<scene name='69/694230/Fadd13_subunits/11'>residues 396-401</scene>) shown in black. Inside the larger N-terminal domain is a <scene name='69/694230/ | + | FadD13 is an ACSVL enzyme that can accept lipids up to 26 carbons as well as being a [http://en.wikipedia.org/wiki/Peripheral_membrane_protein peripheral-membrane protein]<ref>PMID: 20027301</ref>. Unlike other ACSVL proteins, FadD13 is soluble. There are numerous aspects of its structure that affects the way this protein functions. The <scene name='69/694230/Fadd13_subunits/12'>ATP/AMP binding region</scene> is comprised of two separate motifs. The first motif is composed of residues 164-TSGTTGHPKG173-173 shown in red which binds to the phosphate group. The second motif is comprised of residues 298-VQGYALTE-305 shown in blue which binds to the adenine group<ref>DOI: 10.1371/journal.pone.0008387</ref>. Upon binding of ATP or AMP, FadD13 is activated. There are two domains of this protein, a larger N-terminal domain (<scene name='69/694230/Fadd13_subunits/8'>residues 1-395</scene>) shown in blue and a smaller C-terminal domain (<scene name='69/694230/Fadd13_subunits/10'>residues 402-503</scene>) shown in yellow. These domains are held together by a six amino acid linker (<scene name='69/694230/Fadd13_subunits/11'>residues 396-401</scene>) shown in black. Inside the larger N-terminal domain is a <scene name='69/694230/Fadd13_subunits/13'>hydrophobic tunnel</scene> composed of six beta sheets (beta 9-14) shown in green and two alpha helices (alpha 8-9) shown in red.The hydrophobic tunnel allows large lipids/fatty acids, up to 26 carbons, to bind. The tunnel is capped by an arginine and aromatic rich <scene name='69/694230/Hydrophobic_tunnel_beta_12-15/3'>lid loop</scene> shown in yellow that is involved in the peripheral binding of the enzyme to the membrane. Six key arginine residues,<scene name='69/694230/Arginine_surface_patch/3'>ARG 9, 17, 195, 197, 199, 244</scene> create a positively charged surface that is likely involved in initially recruiting FadD13 to the membrane. When these residues were replaced with hyrdrophobic alanine residues, membrane binding increased. This points to the important role of hydrophobic interactions in keeping the protein bound at the membrane<ref>PMID: 22560731</ref>. |
== Function == | == Function == | ||
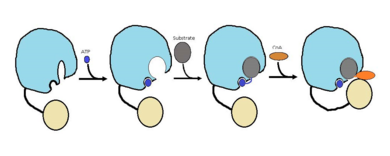
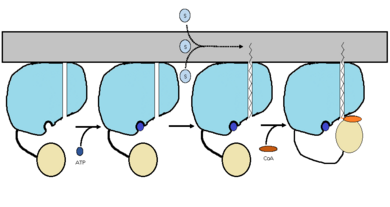
| - | The FadD13 enzyme functions to activate lipids. Once the lipids are activated, they can continue on into metabolic pathways. This is done by ATP/AMP binding to the <scene name='69/694230/Fadd13_subunits/12'>ATP/AMP binding region</scene>. Once ATP/AMP is bound, the long lipid chain up to 26 carbons may bind in the <scene name='69/694230/ | + | The FadD13 enzyme functions to activate lipids. Once the lipids are activated, they can continue on into metabolic pathways. This is done by ATP/AMP binding to the <scene name='69/694230/Fadd13_subunits/12'>ATP/AMP binding region</scene>. Once ATP/AMP is bound, the long lipid chain up to 26 carbons may bind in the <scene name='69/694230/Fadd13_subunits/13'>hydrophobic tunnel</scene> of the enzyme. Upon binding of the substrate, the C terminal swings up to close off the tunnel. From there CoA can bind to produce the final product, an acyl-CoA Thioester. The lipid can now move transversely throughout the membrane and throughout the rest of the cell. Below is the proposed mechanism for ACSVL proteins. |
[[Image:Proposed Mechanism.png|390 px|thumb|left|Figure 2 shows the proposed mechanism for an ACSVL protein bound to the membrane<ref>PMID: 22560731</ref>]] | [[Image:Proposed Mechanism.png|390 px|thumb|left|Figure 2 shows the proposed mechanism for an ACSVL protein bound to the membrane<ref>PMID: 22560731</ref>]] | ||
Revision as of 14:41, 21 April 2015
= FadD13 =
| |||||||||||
References
- ↑ Watkins PA, Maiguel D, Jia Z, Pevsner J. Evidence for 26 distinct acyl-coenzyme A synthetase genes in the human genome. J Lipid Res. 2007 Dec;48(12):2736-50. Epub 2007 Aug 30. PMID:17762044 doi:http://dx.doi.org/M700378-JLR200
- ↑ Kochan G, Pilka ES, von Delft F, Oppermann U, Yue WW. Structural snapshots for the conformation-dependent catalysis by human medium-chain acyl-coenzyme A synthetase ACSM2A. J Mol Biol. 2009 May 22;388(5):997-1008. Epub 2009 Apr 1. PMID:19345228 doi:10.1016/j.jmb.2009.03.064
- ↑ Khare G, Gupta V, Gupta RK, Gupta R, Bhat R, Tyagi AK. Dissecting the role of critical residues and substrate preference of a Fatty Acyl-CoA Synthetase (FadD13) of Mycobacterium tuberculosis. PLoS One. 2009 Dec 21;4(12):e8387. doi: 10.1371/journal.pone.0008387. PMID:20027301 doi:10.1371/journal.pone.0008387
- ↑ Khare G, Gupta V, Gupta RK, Gupta R, Bhat R, Tyagi AK. Dissecting the role of critical residues and substrate preference of a Fatty Acyl-CoA Synthetase (FadD13) of Mycobacterium tuberculosis. PLoS One. 2009 Dec 21;4(12):e8387. doi: 10.1371/journal.pone.0008387. PMID:20027301 doi:10.1371/journal.pone.0008387
- ↑ Andersson CS, Lundgren CA, Magnusdottir A, Ge C, Wieslander A, Molina DM, Hogbom M. The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: Structural Basis for Housing Lipid Substrates Longer than the Enzyme. Structure. 2012 May 2. PMID:22560731 doi:10.1016/j.str.2012.03.012
- ↑ Andersson CS, Lundgren CA, Magnusdottir A, Ge C, Wieslander A, Molina DM, Hogbom M. The Mycobacterium tuberculosis Very-Long-Chain Fatty Acyl-CoA Synthetase: Structural Basis for Housing Lipid Substrates Longer than the Enzyme. Structure. 2012 May 2. PMID:22560731 doi:10.1016/j.str.2012.03.012