We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1061
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
Another highly conserved series of residues is the WSGFRP sequence. This nonpolar sequence is found on the surface of the molecule and is exposed to solvent. [[Image:Hydrophobic region pic.png|thumb| Hydrophobic region WSGFRP on the surface of MtNrdH (red) bound to ligand (green).]]<ref>DOI 10.1002/ijch.201300024</ref> <ref>PMID:21638687</ref> For this reason, it has been hypothesized that this sequence plays a role in the binding of thioredoxin reductase. <ref name="Swastik" />[[Image:Wsgfrpweblogo.png|thumb|center|upright=2.5|Weblogo diagram showing highly conserved WSGFRP region of NrdH in five separate protein structures from ''Nocardiaseriolae'', ''E. coli'', ''Cornebacterium Ammoniagenes'', and ''Mycobacterium Tuberculosis''.]] | Another highly conserved series of residues is the WSGFRP sequence. This nonpolar sequence is found on the surface of the molecule and is exposed to solvent. [[Image:Hydrophobic region pic.png|thumb| Hydrophobic region WSGFRP on the surface of MtNrdH (red) bound to ligand (green).]]<ref>DOI 10.1002/ijch.201300024</ref> <ref>PMID:21638687</ref> For this reason, it has been hypothesized that this sequence plays a role in the binding of thioredoxin reductase. <ref name="Swastik" />[[Image:Wsgfrpweblogo.png|thumb|center|upright=2.5|Weblogo diagram showing highly conserved WSGFRP region of NrdH in five separate protein structures from ''Nocardiaseriolae'', ''E. coli'', ''Cornebacterium Ammoniagenes'', and ''Mycobacterium Tuberculosis''.]] | ||
| - | Arg-68 is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/ | + | <scene name='69/694227/Arg_68/4'>Arg-68</scene> is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/4'>first conformation</scene>, Arg-68 is hydrogen bonded to His- 60 and Asp-59. When Arg-68 shifts to its <scene name='69/694227/Arg_68_conformation_2/5'>second conformation</scene>, it breaks its hydrogen bond with Asp-59. <ref name="Swastik" /> This reduction in hydrogen bonding gives the hydrophobic region more flexibility and is thought to occur when NrdH is in its inactive state. |
| - | , it breaks its hydrogen bond with Asp-59. <ref name="Swastik" /> This reduction in hydrogen bonding gives the hydrophobic region more flexibility and is thought to occur when NrdH is in its inactive state. | + | |
==== Stabilization ==== | ==== Stabilization ==== | ||
Revision as of 23:14, 21 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Structure of Mycobacterium Tuberculosis NrdH
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ 2.0 2.1 "Tuberculosis." Media Centre. World Health Organization, Web. 16 Mar. 2015. Media Centre. <http://www.who.int/mediacentre/factsheets/fs104/en/>.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Nelson, David L., and Michael M. Cox. Lehninger Principles of Biochemistry. 5th ed. New York: W.H. Freeman, 2008. 888-889.
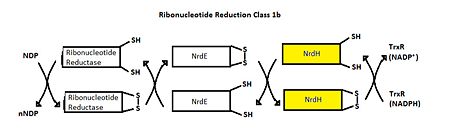
- ↑ Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644