We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1061
From Proteopedia
(Difference between revisions)
| Line 21: | Line 21: | ||
Exactly how this structure relates to function is somewhat debated, but it is hypothesized that the fold allows residues preceding the turn to interact with the CVQC motif after the turn. A threonine-7 reside directly across the thioredoxin fold from the disulfide bond has been suggested to adopt two different conformations which differentially affect the redox abilities of the protein. In the <scene name='69/694228/Nrdh_ligand_binding_site/17'>"A" conformation</scene>, the alcohol oxygen of the threonine side chain (seen as a red ball) points towards the disulfide bond, engaging a electrostatic interaction (represented by a short dashed line) between the two that prevents thioredoxin reductase from binding. Alternatively, in the <scene name='69/694228/Nrdh_ligand_binding_site/16'>"B" Conformation</scene>, the alcohol points in the opposite direction, allowing sufficient space and enough electrostatic freedom for the ligand to bind and reduction to occur.<ref name="Swastik" /> | Exactly how this structure relates to function is somewhat debated, but it is hypothesized that the fold allows residues preceding the turn to interact with the CVQC motif after the turn. A threonine-7 reside directly across the thioredoxin fold from the disulfide bond has been suggested to adopt two different conformations which differentially affect the redox abilities of the protein. In the <scene name='69/694228/Nrdh_ligand_binding_site/17'>"A" conformation</scene>, the alcohol oxygen of the threonine side chain (seen as a red ball) points towards the disulfide bond, engaging a electrostatic interaction (represented by a short dashed line) between the two that prevents thioredoxin reductase from binding. Alternatively, in the <scene name='69/694228/Nrdh_ligand_binding_site/16'>"B" Conformation</scene>, the alcohol points in the opposite direction, allowing sufficient space and enough electrostatic freedom for the ligand to bind and reduction to occur.<ref name="Swastik" /> | ||
| - | Another highly conserved series of residues is the WSGFRP sequence. This nonpolar sequence is found on the surface of the molecule and is exposed to solvent. [[Image:Hydrophobic region pic.png|thumb| Hydrophobic region WSGFRP on the surface of MtNrdH (red) bound to ligand (green). | + | Another highly conserved series of residues is the WSGFRP sequence. This nonpolar sequence is found on the surface of the molecule and is exposed to solvent. [[Image:Hydrophobic region pic.png|thumb| Hydrophobic region WSGFRP on the surface of MtNrdH (red) bound to ligand (green).<ref name="Hanson">DOI 10.1002/ijch.201300024</ref>]] For this reason, it has been hypothesized that this sequence plays a role in the binding of thioredoxin reductase. <ref name="Swastik" />[[Image:Wsgfrpweblogo.png|thumb|center|upright=2.5|Weblogo diagram showing highly conserved WSGFRP region of NrdH in five separate protein structures from ''Nocardiaseriolae'', ''E. coli'', ''Cornebacterium Ammoniagenes'', and ''Mycobacterium Tuberculosis''.]] |
<scene name='69/694227/Arg_68/4'>Arg-68</scene> is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/4'>first conformation</scene>, Arg-68 is hydrogen bonded to His- 60 and Asp-59. When Arg-68 shifts to its <scene name='69/694227/Arg_68_conformation_2/5'>second conformation</scene>, it breaks its hydrogen bond with Asp-59. <ref name="Swastik" /> This reduction in hydrogen bonding gives the hydrophobic region more flexibility and is thought to occur when NrdH is in its inactive state. | <scene name='69/694227/Arg_68/4'>Arg-68</scene> is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/4'>first conformation</scene>, Arg-68 is hydrogen bonded to His- 60 and Asp-59. When Arg-68 shifts to its <scene name='69/694227/Arg_68_conformation_2/5'>second conformation</scene>, it breaks its hydrogen bond with Asp-59. <ref name="Swastik" /> This reduction in hydrogen bonding gives the hydrophobic region more flexibility and is thought to occur when NrdH is in its inactive state. | ||
| Line 37: | Line 37: | ||
== Relevance == | == Relevance == | ||
| - | Like most NrdHs, MtNrdH is similar in sequence to glutaredoxins, but structurally similar to thioredoxins. MtNrdH also accepts electrons from thioredoxin reductase, a characteristic of thioredoxins, but not glutaredoxins.<ref name="Swastik" /> [[Image:Image-Super imposed molecules.png|thumb|left|Structural comparison of NrdHs with "thioredoxin folds": ''E. Coli'' NrdH (green), ''C. ammoniagenes'' NrdH (blue), ''M. tuberculosis'' NrdH (red) | + | Like most NrdHs, MtNrdH is similar in sequence to glutaredoxins, but structurally similar to thioredoxins. MtNrdH also accepts electrons from thioredoxin reductase, a characteristic of thioredoxins, but not glutaredoxins.<ref name="Swastik" /> [[Image:Image-Super imposed molecules.png|thumb|left|Structural comparison of NrdHs with "thioredoxin folds": ''E. Coli'' NrdH (green), ''C. ammoniagenes'' NrdH (blue), ''M. tuberculosis'' NrdH (red)<ref name="Hanson" />]] |
Similar structures of NrdH have been isolated in other primitive species including ''E. coli'', ''S. pyogenes'', ''S. typhimurium'', ''D. deserti'', ''S. flexneri 2457T'', and ''S. dysenteriae''. In higher order multi-cellular organisms, however the NrdH protein is replaced by more complex glutaredoxins or thioredoxins. This observation leads some to speculate that NrdH is one of the very first ancestors in the ribonucleotide reduction pathway. <ref name="Swastik" /> If this is true, NrdH can be seen as a critical protein that allowed for the development of DNA-based life since deoxyribonucleotides could not have existed without the ribonucleotide reduction pathway. A better understanding of the evolutionary timeline of NrdH and similar proteins could shed greater light onto the RNA Wold Hypothesis, specifically describing the time frame of emergence of DNA based life. | Similar structures of NrdH have been isolated in other primitive species including ''E. coli'', ''S. pyogenes'', ''S. typhimurium'', ''D. deserti'', ''S. flexneri 2457T'', and ''S. dysenteriae''. In higher order multi-cellular organisms, however the NrdH protein is replaced by more complex glutaredoxins or thioredoxins. This observation leads some to speculate that NrdH is one of the very first ancestors in the ribonucleotide reduction pathway. <ref name="Swastik" /> If this is true, NrdH can be seen as a critical protein that allowed for the development of DNA-based life since deoxyribonucleotides could not have existed without the ribonucleotide reduction pathway. A better understanding of the evolutionary timeline of NrdH and similar proteins could shed greater light onto the RNA Wold Hypothesis, specifically describing the time frame of emergence of DNA based life. | ||
| Line 43: | Line 43: | ||
==Possible Drug Target== | ==Possible Drug Target== | ||
| - | MtNrdH can serve as a potential drug target to treat tuberculosis. The genes encoding NrdE and NrdF2, a cofactor in class 1b ribonucleotide reduction, are essential for growth of M. tuberculosis in vitro.<ref | + | MtNrdH can serve as a potential drug target to treat tuberculosis. The genes encoding NrdE and NrdF2, a cofactor in class 1b ribonucleotide reduction, are essential for growth of M. tuberculosis in vitro.<ref>Mowa, M. B., et al. (2009) Function and regulation of class I ribonucleotide reductase-encoding genes in mycobacteria. J. Bacteriol. 191 (3), 985−995</ref> This suggest that M. tuberculosis relies solely on class Ib ribonucleotide reduction. If that is the case, NrdH may be an essential gene as well. Since NrdH is not found in humans, a drug that targets NrdH would be able to damage M. tuberculosis cells without hurting the human host. |
</StructureSection> | </StructureSection> | ||
Revision as of 23:36, 21 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
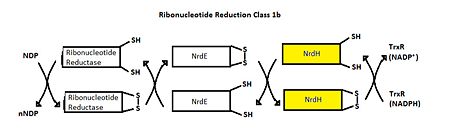
Structure of Mycobacterium Tuberculosis NrdH
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ 2.0 2.1 "Tuberculosis." Media Centre. World Health Organization, Web. 16 Mar. 2015. Media Centre. <http://www.who.int/mediacentre/factsheets/fs104/en/>.
- ↑ 3.0 3.1 Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ 4.0 4.1 Kolberg, M., et al. (2004) Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta 1699 (1−2), 1−34.
- ↑ Nelson, David L., and Michael M. Cox. Lehninger Principles of Biochemistry. 5th ed. New York: W.H. Freeman, 2008. 888-889.
- ↑ Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.
- ↑ Mowa, M. B., et al. (2009) Function and regulation of class I ribonucleotide reductase-encoding genes in mycobacteria. J. Bacteriol. 191 (3), 985−995