We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1056
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
== Active Site == | == Active Site == | ||
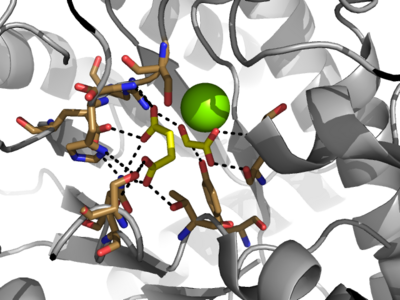
[[Image:Serine_with_active_site.png|400 px|right|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL. Tan molecules represent amino acid side chains. Yellow molecules represent substrates. The magnesium cation is represented by the large, green sphere.]] | [[Image:Serine_with_active_site.png|400 px|right|thumb|Figure 3: Highly ordered hydrogen bonding network within the active site of ICL. Tan molecules represent amino acid side chains. Yellow molecules represent substrates. The magnesium cation is represented by the large, green sphere.]] | ||
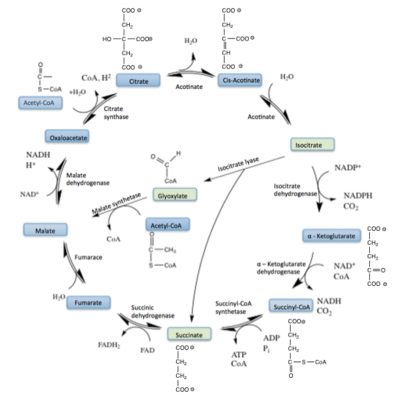
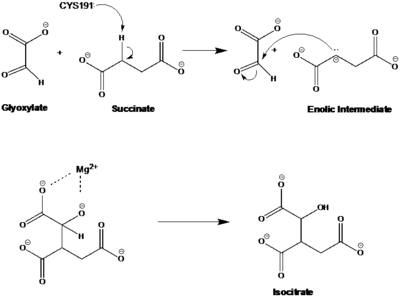
| - | The active site of isocitrate lyase lies near the C-terminal ends of the Beta-strands of the active site<ref name="ICL2"/> | + | The active site of isocitrate lyase lies near the C-terminal ends of the Beta-strands of the active site.<ref name="ICL2"/> The <scene name='69/697526/Binding_pocket/1'>Active Site</scene> is shown here with ligands bound (purple) and catalytic residues shown (gray) in coordination with the magnesium ion (green). The glyoxylate substrate is held into place by a <scene name='69/694223/Serine_and_gly_bond/2'>Network</scene> of hydrogen bonds with Ser 91, Gly 92, Trp 93, and Arg 228<ref name="ICL">PMID:10932251</ref>. The magnesium ion serves to stabilize the partial negative charge placed on the carbonyl oxygens of the glyoxylate. The other substrate, succinate, contains two carboxyl groups and possesses a similar network of hydrogen bonding that holds the ligand in place within the active site. One carboxylate group is hydrogen bound to Asn 313, Glu 295, Arg 228, and Gly 192. The second carboxylate is |
| - | <scene name='69/694223/Second_carboxylate/5'>Hydrogen Bound</scene> to Thr 347, Asn 313, Ser 315, Ser 317, and His 193<ref name="ICL">PMID:10932251</ref> | + | <scene name='69/694223/Second_carboxylate/5'>Hydrogen Bound</scene> to Thr 347, Asn 313, Ser 315, Ser 317, and His 193.<ref name="ICL">PMID:10932251</ref> The ordered hydrogen bonding within the active site orients the succinate molecule such that the alpha carbon is only 3.2 angstroms away from the deprotonated thiol group of Cys 191<ref name="ICL">PMID:10932251</ref><ref name="claisen">Masamune et al. Bio-Claisen condensation catalyzed by thiolase from Zoogloea ramigera. Active site cysteine residues. "Journal of the American Chemical Society" 111: 1879-1881 (1989). DOI: 10.1021/ja00187a053</ref>. |
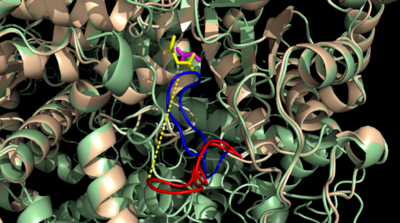
===Catalytic Loop=== | ===Catalytic Loop=== | ||
Revision as of 03:41, 22 April 2015
Isocitrate Lyase from Mycobacterium Tuberculosis
| |||||||||||
3D Structures of Isocitrate Lyase
Updated on 22-April-2015
- ICL from other bacteria
References
- ↑ Srivastava V, Jain A, Srivastava BS, Srivastava R. Selection of genes of Mycobacterium tuberculosis upregulated during residence in lungs of infected mice. Tuberculosis (Edinb). 2008 May;88(3):171-7. Epub 2007 Dec 3. PMID:18054522 doi:http://dx.doi.org/10.1016/j.tube.2007.10.002
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 Sharma V, Sharma S, Hoener zu Bentrup K, McKinney JD, Russell DG, Jacobs WR Jr, Sacchettini JC. Structure of isocitrate lyase, a persistence factor of Mycobacterium tuberculosis. Nat Struct Biol. 2000 Aug;7(8):663-8. PMID:10932251 doi:10.1038/77964
- ↑ 3.0 3.1 3.2 Beeching JR. High sequence conservation between isocitrate lyase from Escherichia coli and Ricinus communis. Protein Seq Data Anal. 1989 Dec;2(6):463-6. PMID:2696959
- ↑ 4.0 4.1 4.2 4.3 Masamune et al. Bio-Claisen condensation catalyzed by thiolase from Zoogloea ramigera. Active site cysteine residues. "Journal of the American Chemical Society" 111: 1879-1881 (1989). DOI: 10.1021/ja00187a053
- ↑ Connely, M. L. Solvent-accessible surfaces of proteins and nucleic acids "Science" 221:709-713 (1983). DOI: 10.1126/science.6879170