Sandbox Reserved 1061
From Proteopedia
(Difference between revisions)
| Line 23: | Line 23: | ||
Exactly how this structure relates to function is somewhat debated, but it is hypothesized that the fold allows residues preceding the turn to interact with the CVQC motif after the turn. A threonine-7 reside directly across the thioredoxin fold from the disulfide bond has been suggested to adopt two different conformations which differentially affect the redox abilities of the protein. In the <scene name='69/694228/Nrdh_ligand_binding_site/17'>"A" conformation</scene>, the alcohol oxygen of the threonine side chain (seen as a red ball) points towards the disulfide bond, forming an electrostatic interaction (represented by a short dashed line) between the two that prevents thioredoxin reductase from binding. Alternatively, in the <scene name='69/694228/Nrdh_ligand_binding_site/16'>"B" Conformation</scene>, the alcohol points in the opposite direction, allowing sufficient space and enough electrostatic freedom for the ligand to bind and reduction to occur.<ref name="Swastik" /> | Exactly how this structure relates to function is somewhat debated, but it is hypothesized that the fold allows residues preceding the turn to interact with the CVQC motif after the turn. A threonine-7 reside directly across the thioredoxin fold from the disulfide bond has been suggested to adopt two different conformations which differentially affect the redox abilities of the protein. In the <scene name='69/694228/Nrdh_ligand_binding_site/17'>"A" conformation</scene>, the alcohol oxygen of the threonine side chain (seen as a red ball) points towards the disulfide bond, forming an electrostatic interaction (represented by a short dashed line) between the two that prevents thioredoxin reductase from binding. Alternatively, in the <scene name='69/694228/Nrdh_ligand_binding_site/16'>"B" Conformation</scene>, the alcohol points in the opposite direction, allowing sufficient space and enough electrostatic freedom for the ligand to bind and reduction to occur.<ref name="Swastik" /> | ||
| - | + | [[Image:Hydrophobic region pic.png|thumb|right| Hydrophobic region WSGFRP on the surface of MtNrdH (red) bound to ligand (green).<ref name="PyMol">The PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC.</ref>]] | |
| + | |||
| + | Another highly conserved series of residues is the WSGFRP sequence. This nonpolar sequence is found on the surface of the molecule and is exposed to solvent. For this reason, it has been hypothesized that this sequence plays a role in the binding of thioredoxin reductase. <ref name="Swastik" /> | ||
<scene name='69/694227/Arg_68/4'>Arg-68</scene> is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/4'>first conformation</scene>, Arg-68 is hydrogen bonded to His- 60 and Asp-59. When Arg-68 shifts to its <scene name='69/694227/Arg_68_conformation_2/5'>second conformation</scene>, it breaks its hydrogen bond with Asp-59. <ref name="Swastik" /> This reduction in hydrogen bonding gives the hydrophobic region more flexibility and is thought to occur when NrdH is in its inactive state. | <scene name='69/694227/Arg_68/4'>Arg-68</scene> is responsible for the stabilization of the hydrophobic region of NrdH. Arg-68 has two distinct conformations. In the <scene name='69/694227/Arg_68_conformation_1/4'>first conformation</scene>, Arg-68 is hydrogen bonded to His- 60 and Asp-59. When Arg-68 shifts to its <scene name='69/694227/Arg_68_conformation_2/5'>second conformation</scene>, it breaks its hydrogen bond with Asp-59. <ref name="Swastik" /> This reduction in hydrogen bonding gives the hydrophobic region more flexibility and is thought to occur when NrdH is in its inactive state. | ||
| + | |||
| + | [[Image:Wsgfrpweblogo.png|thumb|center|upright=2.5|Weblogo diagram showing highly conserved WSGFRP region of NrdH in four separate protein structures from ''Nocardia seriolae'', ''E. coli'', ''Cornebacterium Ammoniagenes'', and ''Mycobacterium Tuberculosis''.<ref name="weblogo" />]] | ||
| + | |||
==== Stabilization ==== | ==== Stabilization ==== | ||
Revision as of 13:18, 24 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
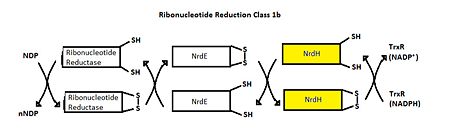
Structure of Mycobacterium Tuberculosis NrdH
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ 2.0 2.1 "Tuberculosis." Media Centre. World Health Organization, Web. 16 Mar. 2015. Media Centre. <http://www.who.int/mediacentre/factsheets/fs104/en/>.
- ↑ 3.0 3.1 Crooks GE, Hon G, Chandonia JM, Brenner SE WebLogo: A sequence logo generator, Genome Research, 14:1188-1190, (2004)
- ↑ 4.0 4.1 The PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC.
- ↑ 5.0 5.1 Kolberg, M., et al. (2004) Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta 1699 (1−2), 1−34.
- ↑ Nelson, David L., and Michael M. Cox. Lehninger Principles of Biochemistry. 5th ed. New York: W.H. Freeman, 2008. 888-889.
- ↑ Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.
- ↑ Wang, M. et al. Mol Cell Proteomics 2012, doi:10.1074/mcp.O111.014704. http://pax-db.org/#!search?q=NrdH%250A
- ↑ Si, M.-R., Zhang, L., Yang, Z.-F., Xu, Y.-X., Liu, Y.-B., Jiang, C.-Y., … Liu, S.-J. (2014). NrdH Redoxin Enhances Resistance to Multiple Oxidative Stresses by Acting as a Peroxidase Cofactor in Corynebacterium glutamicum. Applied and Environmental Microbiology, 80(5), 1750–1762. doi:10.1128/AEM.03654-13
- ↑ Mowa, M. B., et al. (2009) Function and regulation of class I ribonucleotide reductase-encoding genes in mycobacteria. J. Bacteriol. 191 (3), 985−995