We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1061
From Proteopedia
(Difference between revisions)
| Line 38: | Line 38: | ||
MtNrdH has been identified as an electron carrier protein in ribonuleotide reduction. Ribonucleotide reduction uses an enzyme called [http://www.proteopedia.org/wiki/index.php/Ribonucleotide_reductase ribonucleotide reductase (RNR)] to make deoxyribonucleotides, which act as precursors to DNA synthesis. Three classes of RNRs have been identified; each class differs in cofactor requirement, structure, and oxygen dependence, but the general catalytic mechanism is conserved in all three classes.<ref name ="Kolberg">Kolberg, M., et al. (2004) Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta 1699 (1−2), 1−34.</ref> Mycobacterium tuberculosis uses class I ribonucleotide reductase. | MtNrdH has been identified as an electron carrier protein in ribonuleotide reduction. Ribonucleotide reduction uses an enzyme called [http://www.proteopedia.org/wiki/index.php/Ribonucleotide_reductase ribonucleotide reductase (RNR)] to make deoxyribonucleotides, which act as precursors to DNA synthesis. Three classes of RNRs have been identified; each class differs in cofactor requirement, structure, and oxygen dependence, but the general catalytic mechanism is conserved in all three classes.<ref name ="Kolberg">Kolberg, M., et al. (2004) Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta 1699 (1−2), 1−34.</ref> Mycobacterium tuberculosis uses class I ribonucleotide reductase. | ||
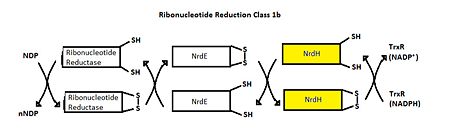
| - | Class I RNR is further subdivided into class Ia and Ib. Both Ia and Ib reduce ribonucleotide 5’ diphosphate to deoxyribonucleotide 5’ diphosphate (NDP to dNDP). | + | Class I RNR is further subdivided into class Ia and Ib. Both Ia and Ib reduce ribonucleotide 5’ diphosphate to deoxyribonucleotide 5’ diphosphate (NDP to dNDP). Ribonucleotide reductase utilizes free thiols to reduce NDP to dNDP. After both of the free thiols give up their electrons, they form a disulfide bond. To be able to perform another round of reduction, the disulfide bond needs to be reduced into free thiols again. In class Ia, RNR is reduced by either glutadoxin or thioredoxin, which are first reduced by glutadoxin reductase and thioredoxin reductase, respectively.<ref>Nelson, David L., and Michael M. Cox. Lehninger Principles of Biochemistry. 5th ed. New York: W.H. Freeman, 2008. 888-889.</ref> In class Ib, RNR is reduced by NrdE, which is first reduced by NrdH (Figure 4). Like ribonucleotide reductase, NrdE and NrdH both use the making and breaking of disulfide bonds to pass electrons down the chain. Thioredoxin reductase uses an NADPH group to reduce NrdH <ref name="Makhlynets" />. An important distinction between Ia and Ib is that Ia is present in eukaryotes, eubacteria, bacteriophages, and virus, but Ib is only present in eubacteria. <ref name="Kolberg" /> |
| - | [[Image:Ribonucleotide Reduction Class 1b.jpg|thumb|center|upright=2.5|'''Figure 4.'''Ribonucleotide Reduction Class Ib general mechanism.<ref>Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.</ref> The role of NrdH is highlighted.]] | + | [[Image:Ribonucleotide Reduction Class 1b.jpg|thumb|center|upright=2.5|'''Figure 4.'''Ribonucleotide Reduction Class Ib general mechanism.<ref name="Makhlynets">Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.</ref> The role of NrdH is highlighted.]] |
== Relevance == | == Relevance == | ||
Revision as of 13:45, 24 April 2015
| This Sandbox is Reserved from 02/09/2015, through 05/31/2016 for use in the course "CH462: Biochemistry 2" taught by Geoffrey C. Hoops at the Butler University. This reservation includes Sandbox Reserved 1051 through Sandbox Reserved 1080. |
To get started:
More help: Help:Editing |
Structure of Mycobacterium Tuberculosis NrdH
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Swastik, Phulera and Mande, Shekhar C. (2013) The Crystal Structure of Mycobacterium tuberculosis NrdH at 0.87Å Suggests a Possible Mode of Its Activity. Biochemistry 52, 4056-4065.
- ↑ 2.0 2.1 "Tuberculosis." Media Centre. World Health Organization, Web. 16 Mar. 2015. Media Centre. <http://www.who.int/mediacentre/factsheets/fs104/en/>.
- ↑ 3.0 3.1 Crooks GE, Hon G, Chandonia JM, Brenner SE WebLogo: A sequence logo generator, Genome Research, 14:1188-1190, (2004)
- ↑ 4.0 4.1 The PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC.
- ↑ 5.0 5.1 Kolberg, M., et al. (2004) Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta 1699 (1−2), 1−34.
- ↑ Nelson, David L., and Michael M. Cox. Lehninger Principles of Biochemistry. 5th ed. New York: W.H. Freeman, 2008. 888-889.
- ↑ 7.0 7.1 Makhlynets, O., Boal, A. K., Rhodes, D. V., Kitten, T., Rosenzweig, A. C., & Stubbe, J. (2014). Streptococcus sanguinis Class Ib Ribonucleotide Reductase: HIGH ACTIVITY WITH BOTH IRON AND MANGANESE COFACTORS AND STRUCTURAL INSIGHTS. The Journal of Biological Chemistry, 289(9), 6259–6272. doi:10.1074/jbc.M113.533554.
- ↑ Wang, M. et al. Mol Cell Proteomics 2012, doi:10.1074/mcp.O111.014704. http://pax-db.org/#!search?q=NrdH%250A
- ↑ Si, M.-R., Zhang, L., Yang, Z.-F., Xu, Y.-X., Liu, Y.-B., Jiang, C.-Y., … Liu, S.-J. (2014). NrdH Redoxin Enhances Resistance to Multiple Oxidative Stresses by Acting as a Peroxidase Cofactor in Corynebacterium glutamicum. Applied and Environmental Microbiology, 80(5), 1750–1762. doi:10.1128/AEM.03654-13
- ↑ Mowa, M. B., et al. (2009) Function and regulation of class I ribonucleotide reductase-encoding genes in mycobacteria. J. Bacteriol. 191 (3), 985−995