Sandbox Reserved 1068
From Proteopedia
(Difference between revisions)
| Line 46: | Line 46: | ||
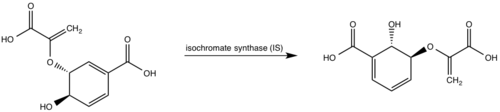
[[Image:CM2.png|450 px|center|thumb|Figure 3: Isochorismate synthase activity <ref>PMID:22307014</ref>.]] | [[Image:CM2.png|450 px|center|thumb|Figure 3: Isochorismate synthase activity <ref>PMID:22307014</ref>.]] | ||
| - | ==Inhibition Studies== | ||
| - | |||
| - | MbtI Inhibition studies aid in the future design of [http://psychology.wikia.com/wiki/Antitubercular_drugs anti-tubercular agents] and [http://en.wikipedia.org/wiki/Broad-spectrum_antibiotic broad-spectrum antibiotics]. Mimics of the enzyme-bound intermediate of MbtI, isochorismate, prove to be significantly more potent inhibitors than the substrate, chorismate mimics <ref name= "1a"/>. Specifically, <scene name='69/694235/3rv6_with_vae1/1'>2-hydroxybenzoate-based inhibitors</scene> that contain extended hydrophobic enol ether side chains at C3 in place of the enol-pyruvate side chain found in chorismate and isochorismate. | ||
== Disease == | == Disease == | ||
[http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis] is the causative agent of [http://www.cdc.gov/tb/ Tuberculosis] (TB), an infectious disease that affects one-third of the worlds population. Two TB-related conditions exist: latent TB infection and active TB disease. Currently, there are four regimens that are approved for the treatment of latent TB infection through the use of the antibiotics isoniazid, rifampin, and rifapentine.TB disease can also be treated through various antibiotic regimens. There are 10 drugs currently approved by the FDA for treating TB disease. The first-line anti-TB agents are the antibiotics isoniazid, rifampin, ethambutol, and pyrazinamide <ref>Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.</ref>. Although various treatments for TB infection and TB disease exist, the emergence of [http://www.cdc.gov/tb/publications/factsheets/drtb/mdrtb.htm multi-drug] and [http://www.cdc.gov/tb/topic/drtb/xdrtb.htm extensively-drug] resistant strains of ''M. tuberculosis'' has increased the need for anti-tubercular agents with novel modes of action. | [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis] is the causative agent of [http://www.cdc.gov/tb/ Tuberculosis] (TB), an infectious disease that affects one-third of the worlds population. Two TB-related conditions exist: latent TB infection and active TB disease. Currently, there are four regimens that are approved for the treatment of latent TB infection through the use of the antibiotics isoniazid, rifampin, and rifapentine.TB disease can also be treated through various antibiotic regimens. There are 10 drugs currently approved by the FDA for treating TB disease. The first-line anti-TB agents are the antibiotics isoniazid, rifampin, ethambutol, and pyrazinamide <ref>Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.</ref>. Although various treatments for TB infection and TB disease exist, the emergence of [http://www.cdc.gov/tb/publications/factsheets/drtb/mdrtb.htm multi-drug] and [http://www.cdc.gov/tb/topic/drtb/xdrtb.htm extensively-drug] resistant strains of ''M. tuberculosis'' has increased the need for anti-tubercular agents with novel modes of action. | ||
| - | [http://en.wikipedia.org/wiki/Iron#Biological_role Iron] is essential for mycobacterial growth and pathogenesis, therefore the pathways for iron acquisition are potential targets for antibacterial therapies.''M. tuberculosis'' obtains iron through two different pathways: chelating iron from the host through the siderophore mycobactin and the degradation of heme released from damaged red blood cells | + | [http://en.wikipedia.org/wiki/Iron#Biological_role Iron] is essential for mycobacterial growth and pathogenesis, therefore the pathways for iron acquisition are potential targets for antibacterial therapies.''M. tuberculosis'' obtains iron through two different pathways: chelating iron from the host through the siderophore mycobactin and the degradation of heme released from damaged red blood cells. |
| + | |||
| + | Mycobactin is a siderophore synthesized by the proteins encoded by the ''mbt'' and ''mbt2'' gene clusters <ref name="5a"/>. The gene Rv2386c is essential for the in vitro growth of "M. tuberculosis" and codes the enzyme MbtI. (turvey, 2010) | ||
| + | |||
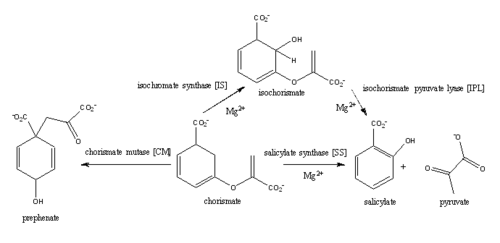
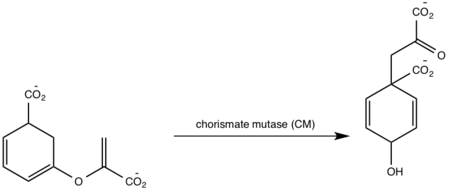
| + | MbtI catalyses the first committed step in the biosynthesis of the siderophore mycobactin and is a potential target for inhibition. The salicylate synthase activity of MbtI produces salicylate and pyruvate from chorismate through an isochorismate intermediate. Inhibition of MbtI activity would decrease the production of salicylate and therefore the synthesis of mycobactin; leading to a decrease in iron acquisition and pathogenesis of ''M. tuberculosis''<ref>De Voss, James J., Kerry Rutter, Benjamin G. Schroeder, Hua Su, and YaQi Zhu. The salicylate-derived mycobacterium siderophore of Mycobacterium tuberculosis are essential for growth in macrophages. "Proceedings of the National Science Academy" 97.3 (2000): 1252-57. Web. 5 Apr. 2015.</ref> . | ||
[[Image:Screen Shot 2015-04-10 at 1.27.15 PM.png|500 px|center|thumb|Figure 3: Reaction catalyzed by MbtI in the mycobactin biosynthesis pathway<ref name= "2a"/>.]] | [[Image:Screen Shot 2015-04-10 at 1.27.15 PM.png|500 px|center|thumb|Figure 3: Reaction catalyzed by MbtI in the mycobactin biosynthesis pathway<ref name= "2a"/>.]] | ||
| + | |||
| + | ==Inhibition Studies== | ||
| + | |||
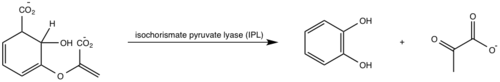
| + | MbtI Inhibition studies aid in the future design of [http://psychology.wikia.com/wiki/Antitubercular_drugs anti-tubercular agents] and [http://en.wikipedia.org/wiki/Broad-spectrum_antibiotic broad-spectrum antibiotics] with a novel mode of action. Mimics of the enzyme-bound intermediate of MbtI, isochorismate, prove to be significantly more potent inhibitors than mimics of the substrate, chorismate <ref name= "1a"/>. The isochorismate mimic were based on a 2,3-dihydroxybenzoate scaffold and proved to be low-micromolar inhibitors of Mbt1 activity. The most potent inhibitors contained hydrophobic enol ether side chains at C3 instead of the enol=pyruvyl side chain seen in chorismate and isochorismate (Turvey 2010) | ||
| + | |||
| + | IsochorismateSpecifically, <scene name='69/694235/3rv6_with_vae1/1'>2-hydroxybenzoate-based inhibitors</scene> that contain extended hydrophobic enol ether side chains at C3 in place of the enol-pyruvate side chain found in chorismate and isochorismate. | ||
</StructureSection> | </StructureSection> | ||
Revision as of 17:19, 25 April 2015
Contents |
Mycobacterium tuberculosis salicylate synthase (Mbt1)
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Chi G, Manos-Turvey A, O'Connor PD, Johnston JM, Evans GL, Baker EN, Payne RJ, Lott JS, Bulloch EM. Implications of Binding Mode and Active Site Flexibility for Inhibitor Potency against the Salicylate Synthase from Mycobacterium tuberculosis. Biochemistry. 2012 Jun 7. PMID:22607697 doi:10.1021/bi3002067
- ↑ 2.0 2.1 doi: https://dx.doi.org/10.1002/cmdc/201000137
- ↑ 3.0 3.1 Manos-Turvey A, Cergol KM, Salam NK, Bulloch EM, Chi G, Pang A, Britton WJ, West NP, Baker EN, Lott JS, Payne RJ. Synthesis and evaluation of M. tuberculosis salicylate synthase (MbtI) inhibitors designed to probe plasticity in the active site. Org Biomol Chem. 2012 Dec 14;10(46):9223-36. doi: 10.1039/c2ob26736e. Epub 2012, Oct 29. PMID:23108268 doi:http://dx.doi.org/10.1039/c2ob26736e
- ↑ Voss, James J., Kerry Rutter, Benjamin G. Schroedor, Hua Su, and YaQi Zhu. "The salicylate-derived mycobactin siderophores of Mycobacterium tuberculosis are essential for growth in macrophages." Proceedings of the National Academy of Sciences 97.3 (2000): 1252-57. Web. 14 Mar. 2015.
- ↑ Lamb AL. Pericyclic reactions catalyzed by chorismate-utilizing enzymes. Biochemistry. 2011 Sep 6;50(35):7476-83. doi: 10.1021/bi2009739. Epub 2011 Aug, 12. PMID:21823653 doi:http://dx.doi.org/10.1021/bi2009739
- ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ 8.0 8.1 8.2 Nicoloff H, Arsene-Ploetze F, Malandain C, Kleerebezem M, Bringel F. Two arginine repressors regulate arginine biosynthesis in Lactobacillus plantarum. J Bacteriol. 2004 Sep;186(18):6059-69. PMID:15342575 doi:http://dx.doi.org/10.1128/JB.186.18.6059-6069.2004
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ He Z, Stigers Lavoie KD, Bartlett PA, Toney MD. Conservation of mechanism in three chorismate-utilizing enzymes. J Am Chem Soc. 2004 Mar 3;126(8):2378-85. PMID:14982443 doi:http://dx.doi.org/10.1021/ja0389927
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Ferrer S, Marti S, Moliner V, Tunon I, Bertran J. Understanding the different activities of highly promiscuous MbtI by computational methods. Phys Chem Chem Phys. 2012 Mar 14;14(10):3482-9. doi: 10.1039/c2cp23149b. Epub, 2012 Feb 3. PMID:22307014 doi:http://dx.doi.org/10.1039/c2cp23149b
- ↑ Tuberculosis (TB). Ed. Sam Posner. Centers for Disease Control and Prevention, n.d. Web. 9 Apr. 2015.
- ↑ De Voss, James J., Kerry Rutter, Benjamin G. Schroeder, Hua Su, and YaQi Zhu. The salicylate-derived mycobacterium siderophore of Mycobacterium tuberculosis are essential for growth in macrophages. "Proceedings of the National Science Academy" 97.3 (2000): 1252-57. Web. 5 Apr. 2015.
Student contributors
Stephanie Raynor Robin Gagnon